��2009?ɽ��������ѧ-���ʽṹ�����ʣ�C��SiԪ���ڻ�ѧ��ռ�м�����Ҫ�ĵ�λ��
��1��д��Si�Ļ�̬ԭ�Ӻ�������Ų�ʽ
1s22s22p63s23p2
1s22s22p63s23p2
��
�ӵ縺�ԽǶȷ�����C��Si��OԪ�صķǽ�����������ǿ������˳��Ϊ
O��C��Si
O��C��Si
��
��2��SiC�ľ���ṹ�뾧�������ƣ�����Cԭ�ӵ��ӻ���ʽΪ
sp3
sp3
��������ڵ���������
���ۼ�
���ۼ�
��
��3��������MO�ĵ���������SiC����ȣ���MΪ
Mg
Mg
����Ԫ�ط��ţ���MO�����������²��ϣ��侧��ṹ��NaCl�������ƣ�MO���۵��CaO�ĸߣ���ԭ����
Mg2+�뾶��Ca2+С��MgO�����ܴ�
Mg2+�뾶��Ca2+С��MgO�����ܴ�
��
��4��C��SiΪͬһ�����Ԫ�أ�CO
2��SiO
2��ѧʽ���ƣ����ṹ�������кܴ�ͬ��CO
2��C��Oԭ�Ӽ��γɦҼ��ͦм���SiO
2��Si��Oԭ�Ӽ䲻�γ������н�����ԭ�Ӱ뾶��С�ĽǶȷ�����Ϊ��C��Oԭ�Ӽ����γɣ���Si��Oԭ�Ӽ䲻���γ������м�
Si��ԭ�Ӱ뾶�ϴ�Si��Oԭ�Ӽ����ϴ�p-p����粢���ص��̶Ƚ�С�������γ������ȶ��Ħм�
Si��ԭ�Ӱ뾶�ϴ�Si��Oԭ�Ӽ����ϴ�p-p����粢���ص��̶Ƚ�С�������γ������ȶ��Ħм�
��



 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
 ��2009?ɽ����ij�¶��£���ͬpHֵ������ʹ�����Һ�ֱ��ˮϡ�ͣ�ƽ��pHֵ����Һ����仯����������ͼ��ʾ����ͼ�ж���ȷ���ǣ�������
��2009?ɽ����ij�¶��£���ͬpHֵ������ʹ�����Һ�ֱ��ˮϡ�ͣ�ƽ��pHֵ����Һ����仯����������ͼ��ʾ����ͼ�ж���ȷ���ǣ�������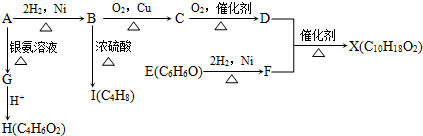
 CH3CH2CH2-
CH3CH2CH2- CH3CH2CH2-
CH3CH2CH2-