
| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��DΪ��ѧ���������嵥�ʣ��ס��ҡ�������Ϊ�����Ļ������ˮ��Һ�ʼ��ԣ�����֮����������ʾת����ϵ�����ֲ��P��Ӧ��������ȥ��
��֪A��B��DΪ��ѧ���������嵥�ʣ��ס��ҡ�������Ϊ�����Ļ������ˮ��Һ�ʼ��ԣ�����֮����������ʾת����ϵ�����ֲ��P��Ӧ��������ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

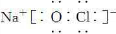
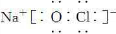
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������к�����������һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
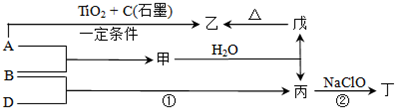
��֪A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1 mol�������в�ͬԭ�ӵ���Ŀ��Ϊ1��2���Һ���18 mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���á������ʼ��ת����ϵ����ͼ��ʾ(ijЩ��������ȥ)��

��ش𣺣�
(1)����B�����Ԫ�������ڱ��е�λ���� ��
(2)��Ļ�ѧʽΪ ������ǿ�Ӧ�����ӷ���ʽ�� ��
(3)NaClO�ĵ���ʽΪ ��
(4)����ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
(5)��Ӧ���У�0.5molNaClO�μӷ�Ӧʱ��ת��1mol���ӣ��仯ѧ����ʽΪ�� ��
(6)һ�������£�A��TiO2��C(ʯī)��Ӧֻ�����Һ�̼����(TiC)�����߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֡���֪�÷�Ӧ����1mol��ʱ�ų�536 kJ���������Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ������ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ������
��11 �֣���֪ A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1 mol �������в�ͬԭ�ӵ���Ŀ��Ϊ1 ��2���Һ���18 mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���á������ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ����

��ش𣺣�1������B�����Ԫ�������ڱ��е�λ����_________��
��2����Ļ�ѧʽΪ________������ǿ�Ӧ�����ӷ���ʽ��________________
��3�������������Ļ�ѧ��������________ ������ĸ��ţ���
a�����Ӽ� b�����Թ��ۼ� c���Ǽ��Թ��ۼ�
��4����Ӧ�ٵĻ�ѧ����ʽΪ________________________��
��5����Ӧ���У�0��5mol NaClO�μӷ�Ӧʱ��ת��1 mol���ӣ��仯ѧ����ʽΪ_________
��6�����������£�A��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC�������߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֡���֪���÷�Ӧ����1 mol��ʱ�ų�536 kJ���������Ȼ�ѧ����ʽΪ_______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com