
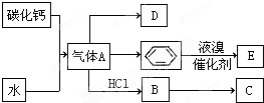
| A�� | ˮ���ӵĻ�ѧ���ʸı� | B�� | ˮ���Ӽ�ṹ���������ʸı� | ||
| C�� | ˮ���Ӽ���������С | D�� | ˮ����������������� |
���� С������ˮ�����Է���Ϊ������λ��ɵľۼ��壬���Է��ӽṹ��û�иı䣬�����е���������û�����̣���ѧ���ʸ�����ı䣮���ı���Ƿ��Ӽ�Ľṹ�����Ӽ���������ǿ���������ʸı䣮
��� �⣺A��С������ˮ�����Է���Ϊ������λ��ɵľۼ��壬��ѧ������ˮ��������ˮ�Ļ�ѧ����һ��������û�иı䣬��A����
B��������ˮ���Ӽ�ľ����С��ˮ���Ӽ�ṹ�ı䣬���Ӽ���������ǿ���е����ߣ��ܶ�����B��ȷ��
C��С������ˮ���Ӽ�ľ����С�����Ӽ���������ǿ����C����
D��С������ˮֻ�Ǹı�ˮ���Ӽ������������ѧ��û�иı䣬�����е���������û�����̣���D����
��ѡB��
���� ��������Ϣ����ʽ����ˮ�еĻ�ѧ�������Ӽ���������ע������Ϣ����ѧ֪ʶ����ɣ���Ŀ�ѶȲ���ѡ��CΪ�����״�����ѵ㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3��Һ�м���ϡ HCl��CO23-+2H+�TH2O+CO2�� | |
| B�� | �� NaHSO4�� Ba��OH��2��Һ��������ԣ�2H++SO24-+Ba2++2OH-�TBaSO4��+2H2O | |
| C�� | ��������Һ��ͨ������ CO2���壺2C6H5O-+CO2+H2O��2C6H5OH+CO23- | |
| D�� | �ö��Ե缫����Ȼ�����Һ��2Cl-+2H2O�TCl2��+H2��+2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KCl���� | B�� | K2SO4��Һ | C�� | ���� | D�� | KNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2CH2CH3 | B�� | CH3CH��CH3��CH3 | C�� | C��CH3��4 | D�� | CH3CH��CH3��CH2CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��pH��ֽ�����ˮ��pH=2���ô�ĥ�ڲ��������Լ�ƿ����K2CO3��Һ | |
| B�� | ����ʽ�ζ��ܻ���Һ����ȡ20.00 mL����K2Cr2O7��Һ | |
| C�� | ��������ƽ��ȡ5.85 g NaNO3���壻��10mL��Ͳ��ȡ6.8mLϡH2SO4 | |
| D�� | ����к͵ζ�ʱ���ζ��ܺ���ƿ�����ñ�Һ�����Һ��ϴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4��CH3CH3 | B�� | 11H�� 12H | C�� | O2��O3 | D�� | D2O��T2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�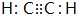 ��D�����ʽΪCH2��
��D�����ʽΪCH2��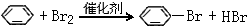 ���䷴Ӧ����Ϊȡ����Ӧ��
���䷴Ӧ����Ϊȡ����Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��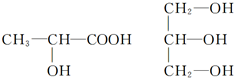
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 2.4g ����þ��Ϊþ����ʱʧȥ�ĵ�����Ϊ0.1NA | |
| B�� | 1mol�����������������Ϊ10NA | |
| C�� | ���³�ѹ�£�2g�������еķ�����32g�����з�����Ŀ��ΪNA | |
| D�� | 4g��������ԭ����Ϊ2NA |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com