
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺| 0.9 |
| Mr |
| 1.12 |
| 22.4 |
| 0.9 |
| Mr |
| 1.12 |
| 22.4 |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L(��״��)����ش��������⣺
��1��BԪ����Ԫ�����ڱ��е�λ���� ��
��2��A��B��C����Ԫ������������Ӧ��ˮ�������Խ�ǿ�����Խ�����˳����(д��ѧʽ)
��
��3��A��B��Ԫ�ص�����������Ӧ��ˮ����֮����Է�����Ӧ�������ӷ���ʽ
��
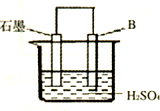
��4����B������ʯī������ͼװ�����ӣ�ʯī�缫������Ϊ ���õ缫��ӦʽΪ�� ��
��5��C��һ���������dz����Ĵ�����Ⱦ�Ϊ��ֹ������Ⱦ��ij��������NaOH��Һ��ʯ�Һ�O2������C�������������β����ʹ��ת��Ϊʯ�ࣨCaSO4��2H2O����������ת��������CԪ�ز���ʧ��ÿ�촦��1120 m3����״���£���2%����������������������β���������Ͽ��Եõ�����ǧ��ʯ�ࣨ��д����Ҫ�ļ�����̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L(��״��)����ش��������⣺
��1��BԪ����Ԫ�����ڱ��е�λ����
��2��A��B��C����Ԫ������������Ӧ��ˮ�������Խ�ǿ�����Խ�����˳����(д��ѧʽ)
��3��A��B��Ԫ�ص�����������Ӧ��ˮ����֮����Է�����Ӧ�������ӷ���ʽ
Ϊ
��4����B������ʯī����ͼװ�����ӣ�ʯī�缫������Ϊ
����һ�缫��ӦʽΪ�� ��
��5��C��һ���������dz����Ĵ�����Ⱦ�Ϊ��ֹ������Ⱦ��ij��������NaOH��Һ��ʯ�Һ�O2������C�������������β����ʹ��ת��Ϊʯ�ࣨCaSO4��2H2O����������ת��������CԪ�ز���ʧ��ÿ�촦��1120 m3����״���£���2%����������������������β���������Ͽ��Եõ�����ǧ��ʯ�ࣿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ���Ƹ���ѧ��һ�ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ�������
��10�֣�����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L(��״��)����ش��������⣺
��1��BԪ����Ԫ�����ڱ��е�λ���� ��
��2��A��B��C����Ԫ������������Ӧ��ˮ�������Խ�ǿ�����Խ�����˳����(д��ѧʽ)
��
��3��A��B��Ԫ�ص�����������Ӧ��ˮ����֮����Է�����Ӧ�������ӷ���ʽ
��
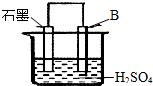
��4����B������ʯī������ͼװ�����ӣ�ʯī�缫������Ϊ ���õ缫��ӦʽΪ�� ��
��5��C��һ���������dz����Ĵ�����Ⱦ�Ϊ��ֹ������Ⱦ��ij��������NaOH��Һ��ʯ�Һ�O2������C�������������β����ʹ��ת��Ϊʯ�ࣨCaSO4��2H2O����������ת��������CԪ�ز���ʧ��ÿ�촦��1120 m3����״���£���2%����������������������β���������Ͽ��Եõ�����ǧ��ʯ�ࣨ��д����Ҫ�ļ�����̣���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com