
 ������ͼ����֮���ת����ϵ���ش�
������ͼ����֮���ת����ϵ���ش�| Fe |
| ϡ���� |
| H2 |
| NaOH��Һ |
| �� |
| Fe |
| ϡ���� |
| H2 |
| NaOH��Һ |
| �� |


 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
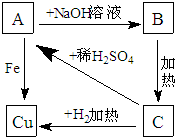
��һ�������¿�ʵ����ͼ��ʾ����֮����ת�䣺
(1)��������Ʋ⣬����д�������ʵĻ�ѧʽ��
A��______��B��______��
D��______��G��______��
(2)��Ҫ˵��A��ת��ΪB��C��ԭ��д���йط�Ӧ�Ļ�ѧ����ʽ��
___________________________________________________
(3)д��E������A��Һ��Ӧ�����ӷ�Ӧ����ʽ��______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��16�֣���һ��������ܱ����м���2 mol A��0��6 mo1 C��һ������B�������壮һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ(��)��ʾ������t0��t1 ��c(B)δ������ͼ(��)Ϊt2ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ��¶ȡ�ѹǿ�������е�һ������������t3��t4��Ϊʹ�ô�����
��ش��������⣺
(1)��t1��15 min����t0��t1����C���ʵ�Ũ�ȱ仯��ʾ��Ӧ����Ϊ ��
(2) t4��t5�θı������Ϊ ��B����ʼ���ʵ���Ũ��Ϊ ������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
| t1��t2 | t2��t3 | t3��t4 | t4��t5 | t5��t6 |
| K1 | K2 | K3 | K4 | K5 |
��K1= (������λС��)��
K1��K2��K3��K4��K5֮��Ĺ�ϵΪ (�á�>������<����������)��
(3) t5��t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01 mol�����˹����������������Ƚ�������ΪakJ��д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ
��
(4)����ͬ�����£�����ʼʱ�����м���a mol A��b mol B��c mol C��Ҫ�ﵽt1ʱ��ͬ����ƽ�⣬a��b��cҪ���������Ϊ .
����������1����ѧ��Ӧ����ͨ���õ�λʱ����Ũ�ȵı仯������ʾ������ͼ��I��֪��15min��C��Ũ�ȱ仯����0.6mol/L��0.3mol/L��0.3mol/L����������Ϊ
��2����ͼ����֪����t4��t5�η�Ӧ���ʼ�С����ƽ�ⲻ�ƶ������Ըı���������������¶Ⱥ�Ũ�ȣ�������t3��t4��Ϊʹ�ô��������Ըý�ֻ�ǽ���ѹǿ����˵���ı�ѹǿƽ�ⲻ�ƶ�����˷�Ӧǰ������Dz���ġ�����ͼ��I��֪A�Ƿ�Ӧ�C����������ߵı仯��֮����2�U3������Ҫʹ��Ӧǰ��������䣬Bֻ�Ƿ�Ӧ�����Ӧ�ķ���ʽΪ2A��B3C��C��Ũ��������0.3mol/L����B��Ũ�ȼ�����0.1mol/L������B����ʼŨ����0.4mol/L��0.1mol/L��0.5mol/L����t1��t2��ƽ����ABC��Ũ�ȣ�mol/L���ֱ�Ϊ0.8��0.4��0.6������ƽ�ⳣ��Ϊ
![]() ����Ϊƽ�ⳣ��ֻ���¶��йأ�����ͼ���֪��t5��t6�Σ����淴Ӧ����ͬʱ����ƽ��������Ӧ�����ƶ�����Ϊ������ֻ�����¶����������¶ȣ����Է�Ӧ�����ȷ�Ӧ�����K1��K2��K3��K4��K5֮��Ĺ�ϵΪK1=K2=K3=K4<K5��
����Ϊƽ�ⳣ��ֻ���¶��йأ�����ͼ���֪��t5��t6�Σ����淴Ӧ����ͬʱ����ƽ��������Ӧ�����ƶ�����Ϊ������ֻ�����¶����������¶ȣ����Է�Ӧ�����ȷ�Ӧ�����K1��K2��K3��K4��K5֮��Ĺ�ϵΪK1=K2=K3=K4<K5��
��3������������жϣ�ÿ����0.01molA����Ӧ����������akJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ2A(g)+B(g)⇋3C(g) ��H����200kJ/mol��
��4����Ϊ�����ݻ����䣨�����ݻ���2L��������Ҫʹƽ���Ч������ʼ���ʵ����ʵ�������ȵġ����������֪��ʼʱAB�����ʵ����ֱ�Ϊ2.4mol��1.2mol�����cmol��Cת��ΪAB����AB�����ʵ�����mol���ֱ���a��2c/3��b��c/3�����Թ�ϵʽ��������a��2c/3��2.4��b��c/3��1.2.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ�߶���ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ������
��16�֣� ��һ��������ܱ����м���2 mol A��0��6 mo1 C��һ������B�������壮һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ(��)��ʾ������t0��t1 ��c(B)δ������ͼ(��)Ϊt2ʱ�̺�ı䷴Ӧ��������ѧ��Ӧ������ʱ��仯��������ĸ��θı������������ͬ��ÿ����ֻ�ı�Ũ�ȡ��¶ȡ�ѹǿ�������е�һ������������t3��t4��Ϊʹ�ô�����

��ش��������⣺
(1)��t1��15 min����t0��t1����C���ʵ�Ũ�ȱ仯��ʾ��Ӧ����Ϊ ��
(2) t4��t5�θı������Ϊ ��B����ʼ���ʵ���Ũ��Ϊ ������ƽ��ʱ��Ӧ��ƽ�ⳣ�����±���ʾ��
|
t1��t2 |
t2��t3 |
t3��t4 |
t4��t5 |
t5��t6 |
|
K1 |
K2 |
K3 |
K4 |
K5 |
��K1= (������λС��)��
K1��K2��K3��K4��K5֮��Ĺ�ϵΪ (�á�>������<����������)��
(3) t5��t6�α����������¶Ȳ��䣬��A�����ʵ������仯��0.01 mol�����˹����������������Ƚ�������ΪakJ��д�����¶��¸÷�Ӧ���Ȼ�ѧ����ʽ
��
(4)����ͬ�����£�����ʼʱ�����м���a mol A��b mol B��c mol C��Ҫ�ﵽt1ʱ��ͬ����ƽ�⣬a��b��cҪ���������Ϊ .
����������1����ѧ��Ӧ����ͨ���õ�λʱ����Ũ�ȵı仯������ʾ������ͼ��I��֪��15min��C��Ũ�ȱ仯����0.6mol/L��0.3mol/L��0.3mol/L����������Ϊ
��2����ͼ����֪����t4��t5�η�Ӧ���ʼ�С����ƽ�ⲻ�ƶ������Ըı���������������¶Ⱥ�Ũ�ȣ�������t3��t4��Ϊʹ�ô��������Ըý�ֻ�ǽ���ѹǿ����˵���ı�ѹǿƽ�ⲻ�ƶ�����˷�Ӧǰ������Dz���ġ�����ͼ��I��֪A�Ƿ�Ӧ�C����������ߵı仯��֮����2�U3������Ҫʹ��Ӧǰ��������䣬Bֻ�Ƿ�Ӧ�����Ӧ�ķ���ʽΪ2A��B 3C��C��Ũ��������0.3mol/L����B��Ũ�ȼ�����0.1mol/L������B����ʼŨ����0.4mol/L��0.1mol/L��0.5mol/L����t1��t2��ƽ����ABC��Ũ�ȣ�mol/L���ֱ�Ϊ0.8��0.4��0.6������ƽ�ⳣ��Ϊ
3C��C��Ũ��������0.3mol/L����B��Ũ�ȼ�����0.1mol/L������B����ʼŨ����0.4mol/L��0.1mol/L��0.5mol/L����t1��t2��ƽ����ABC��Ũ�ȣ�mol/L���ֱ�Ϊ0.8��0.4��0.6������ƽ�ⳣ��Ϊ ����Ϊƽ�ⳣ��ֻ���¶��йأ�����ͼ���֪��t5��t6�Σ����淴Ӧ����ͬʱ����ƽ��������Ӧ�����ƶ�����Ϊ������ֻ�����¶����������¶ȣ����Է�Ӧ�����ȷ�Ӧ�����K1��K2��K3��K4��K5֮��Ĺ�ϵΪK1=K2=K3=K4<K5��
����Ϊƽ�ⳣ��ֻ���¶��йأ�����ͼ���֪��t5��t6�Σ����淴Ӧ����ͬʱ����ƽ��������Ӧ�����ƶ�����Ϊ������ֻ�����¶����������¶ȣ����Է�Ӧ�����ȷ�Ӧ�����K1��K2��K3��K4��K5֮��Ĺ�ϵΪK1=K2=K3=K4<K5��
��3������������жϣ�ÿ����0.01molA����Ӧ����������akJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ2A(g)+B(g)⇋3C(g) ��H����200kJ/mol��
��4����Ϊ�����ݻ����䣨�����ݻ���2L��������Ҫʹƽ���Ч������ʼ���ʵ����ʵ�������ȵġ����������֪��ʼʱAB�����ʵ����ֱ�Ϊ2.4mol��1.2mol�����cmol��Cת��ΪAB����AB�����ʵ�����mol���ֱ���a��2c/3��b��c/3�����Թ�ϵʽ��������a��2c/3��2.4��b��c/3��1.2.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com