
���ֶ�����Ԫ��A��B��C��D��E��ԭ������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ��������ӻ�������ҡ�Aԭ�ӵ������������ȴ������Ӳ��3����E������������Eԭ������������������������Ϣ�ش��������⣺
��1��A��B��C��D��E����Ԫ�ص�ԭ�Ӱ뾶��С�����˳���� ����Ԫ�ط�����д��
��2���������д��ڵĻ�ѧ�������� ��
��3��ѡ��ǡ�����Լ���ȥD��������E��д��������Ӧ�Ļ�ѧ����ʽ
��4���������ҵĵ���ʽ
��5����D��E�ĵ��ʲ���NaOH��Һ�У�����ԭ��أ��为����Ӧ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H��H����Ϊ436 KJ/mol��H��N����Ϊ391KJ/mol�����ݻ�ѧ����ʽ��
N2 �� 3H2  2NH3 ��H����92.4 KJ/mol����N��N���ļ����� ( )
2NH3 ��H����92.4 KJ/mol����N��N���ļ����� ( )
A��431 KJ/mol B��946 KJ/mol C��649 KJ/mol D��869 KJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A��B��C��D��E��F��G��H�ת����ϵ����ͼ��ʾ��5.2 g F����100 mL 1 mol/L NaOH��Һǡ����ȫ�кͣ�0.1 mol F����������NaHCO3��Ӧ���ڱ�״���·ų�4.48 L CO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���
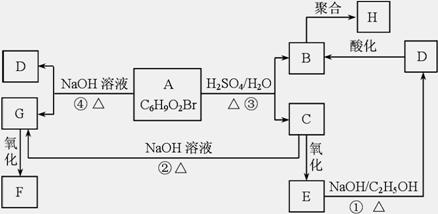
(1)д������C�еĹ����ŵ����ƣ� ��
(2)д������F��H�Ľṹ��ʽ��
F ��H ��
(3)д����Ӧ�١��ܵĻ�ѧ��Ӧ���ͣ��� ���� ��
(4)д���仯�١��۵Ļ�ѧ����ʽ��
��
��
(5)д����Է���������B��14������B������ͬ�����ŵ��������ʵĽṹʽ�������������칹����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����пƬ�ʹ�ͭƬ��ͼ��ʽ����ͬŨ�ȵ�ϡ������һ��ʱ�䣬����������ȷ����
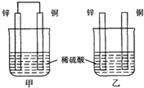 A�����ձ�����Һ��pH������
A�����ձ�����Һ��pH������
B������ͭƬ������������ͭƬ�Ǹ���
C�����ձ���ͭƬ����������ݲ���
D���ס�����Һ������ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
a mL������̬����ɵĻ����������������ϣ���ȼ��ը�ָ���ԭ����״̬(���¡���ѹ)���������С2a mL����������������
A��CH4��C2H4��C3H4 B��C2H6��C3H6��C4H6
C��CH4��C2H6��C3H8 D��C2H4��C2H2��CH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
0.2 mol�л����0.4 mol O2���ܱ�������ȼ�պ����ΪCO2��CO��H2O(g)�����ᆳ��ŨH2SO4����������10.8 g����ͨ�����ȵ�CuO����ַ�Ӧ��CuO��������3.2 g�����������ͨ����ʯ�ұ���ȫ���գ���������17.6 g������ʾ��ͬһ��Cԭ���������ǻ��Dz��ȶ��ṹ��ͨ�������������������⣬Ҫ�б�Ҫ������̣�
(1)���ƶϸ��л���ķ���ʽ��
(2)��0.2 mol���л���ǡ������9.2 g��������ȫ��Ӧ����ȷ�����л���Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11.2L����״������ϩ������Ļ������ͨ����������ˮ�У���ַ�Ӧ����ˮ������������5.6g����ԭ������������ϩ����������ʵ���֮�Ⱥ�����֮�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о�С��Ϊ��̽��һ����������X(��������Ԫ��)����ɺ����ʣ���Ʋ����������ʵ�飺

��ȡ10.80 g X�ڶ��������м�������ȫ�ֽ⣬�õ�6.40 g����1����ش��������⣺
(1)������ɫ����1�н���Ԫ�ص�ԭ�ӽṹʾ��ͼ��________________��д������ĵ���ʽ��________��
(2)X�Ļ�ѧʽ��________���ڶ��������м���X����ȫ�ֽ�Ļ�ѧ����ʽΪ__________________________________________________________��
(3)��ɫ����2�ڿ����б�ɺ��ɫ������ԭ����______________________________(�û�ѧ��Ӧ����ʽ��ʾ)��
(4)һ�������£�����������1�е�ij�ֳɷֿ��ܷ���������ԭ��Ӧ��д��һ�����ܵĻ�ѧ��Ӧ����ʽ��_____________________________________________________�������ʵ�鷽����֤�÷�Ӧ�IJ��__________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com