
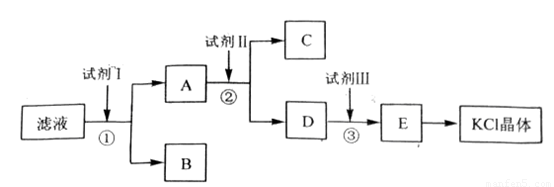
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����
�ش��������⣺
��1����ʼ��Һ��pH_____7(����ڡ���С�ڡ����ڡ�)����ԭ����______________________________��(�����ӷ���ʽ��ʾ)
��2���Լ���Ļ�ѧʽΪ___________�����з�����Ӧ�����ӷ���ʽΪ_______________��
��3���Լ���Ļ�ѧʽΪ____________�����м����Լ����Ŀ����__________________��
��4���Լ����������_____________�����з�����Ӧ�����ӷ���ʽΪ________________��
��1������CO32-+H2O HCO3- +OH-
(HCO3-+H2O
HCO3- +OH-
(HCO3-+H2O H2CO3+ OH-δд���۷�)
H2CO3+ OH-δд���۷�)
��2��BaCl2 Ba2++SO42-=BaSO4 ��Ba2++CO32��=BaCO3��д���۷֣�
��3��K2CO3 ��ȥ������Ba2+
��4������ CO32�� +2H+=H2O+CO2
��������
�����������1����ʼ��Һ�к���̼��أ�̼���ˮ��ʼ��ԣ�����Һ��PH����7����2��Ҫ�������������������̼�����Ӧ��������ı����ӣ���3��Ҫ��������ı����ӣ�Ҫ����̼��أ���4��Ҫ���������̼�����Ҫ�μ����������
���㣺��Һ������Ե�ԭ���ӵķ��������衢���ӷ���ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

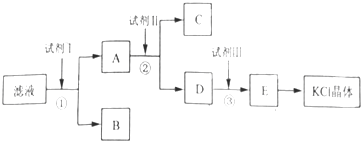
(1)������Ʒ����ʱ����Ӧ�Ļ�ѧ����ʽΪ___________________��
(2)�ܷ���Ba(NO3)2����BaCl2________,������_______________________________��
(3)֤��![]() ������ȫ�ķ�����___________________________________________��
������ȫ�ķ�����___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���¿α���������¿����ģ���ѧ�Ծ���A�����������棩 ���ͣ��ƶ���
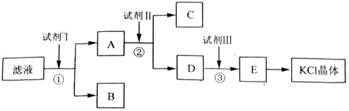
[2012��ȫ����]��12�֣��Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����
�ش��������⣺
��1����ʼ��Һ��pH_ _7(����ڡ�����С�ڡ����ڡ�)����ԭ����____________��
��2���Լ���Ļ�ѧʽΪ______�����з�����Ӧ�����ӷ���ʽΪ___________ ______��
��3���Լ���Ļ�ѧʽΪ______�����м����Լ����Ŀ����____________ ________��
��4���Լ����������________�����з�����Ӧ�����ӷ���ʽΪ_________ ____��
��5��ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.775 9 g���ܽ������100 mL����ƿ�У�ÿ��ȡ25.00 mL��Һ����0.100 0 mol��L��1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62 mL���ò�Ʒ�Ĵ���Ϊ_________________________________(��ʽ��������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com