
(1)�±�Ϊϩ��������巢���ӳɷ�Ӧ���������(����ϩΪ��)��
| ϩ����� | ������� |
| (CH3)2C==CHCH3 | 10.4 |
| CH3CH==CH2 | 2.03 |
| CH2== CH2 | 10.0 |
| CH2==CHBr | 0.04 |
�ݱ������ݣ��ܽ�ϩ��������ʱ����Ӧ������C==C��ȡ���������ࡢ������Ĺ�ϵ��____________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������_________��
A.(CH3)2C==C(CH3)2
B.CH3CH==CHCH3
C.CH2== CH2
D.CH2==CHCI
(1)��C==C�����м�(���)�������ڼӳɷ�Ӧ���ڼ�(���)Խ�࣬����Խ��C==C��������(±��)�������ڼӳɷ�Ӧ��(2)D
����Ϊ��Ϣ�����⣬��Ҫ�����Ķ���������ѧ�������ۺϷ���������˼ά��Ǩ�ƺͷ�ɢ������
(1)�������Ϣ��֪��ϩ���������ӳɵķ�Ӧ����(CH3)2C==CHCH3>CH3CH==CH2>CH2==CH2���Ա����ǵĽṹ�ó����ۣ�C==C�����м��������ڼӳɷ�Ӧ����Խ�࣬����Խ���ɶԱ�CH2==CH2��CH2==CHBr�ֱ�����ӳɵķ�Ӧ���ʵĴ�С��˵��C==C��������ԭ��ʱ�������ڼӳɷ�Ӧ��(2)ϩ��������Ȼ���ӳ����ʴ�СӰ�������(1)�й������ơ��ڸ���������ϩ������У�CH2==CHCI�ṹ��C==C�������ӣ���������ԭ�ӣ����Ȼ���ӳ�������С�����Դ�ΪD��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�±�Ϊϩ��������巢���ӳɷ�Ӧ���������(����ϩΪ��)��
ϩ����� | ������� |
(CH3) | 10.4 |
CH3CH=CH2 | 2.03 |
CH2=CH2 | 1.00 |
CH2=CHBr | 0.04 |
�ݱ������ݣ��ܽ�ϩ��������ʱ����Ӧ������C=C��ȡ���������ࡢ������Ĺ�ϵ��_____________________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������_________(�����)��
A.(CH3)2C=C(CH3)2 B.CH3CH=CHCH3
C.CH2=CH2 D.CH2=CHCl
(3)ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
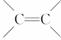
(��Ҫ����) (��Ҫ����)
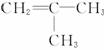
(��Ҫ����) (��Ҫ����)
���п�ͼ��C�Ľṹ��ʽΪ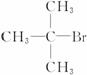 ���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ�
���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ�
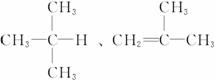
������ͼ�У�B�Ľṹ��ʽΪ____________________________________������ȡ����Ӧ����_________(���ͼ�����)��������ȥ��Ӧ����________(�����)��д��A��D�Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩ����� | ������� |
(CH3)2C=CHCH3 | 10.4 |
CH3CH=CH2 | 2.03 |
CH2=CH2 | 1.00 |
CH2=CHBr | 0.04 |
�ݱ������ݣ��ܽ�ϩ���������ӳ�ʱ����Ӧ������C=C��ȡ���������ࡢ������Ĺ�ϵ��_________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������_________(�����)��
A.(CH3)2C=CHCH3 B.CH3CH=CH2
C.CH2=CH2 D.CH2=CHBr
(3)ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
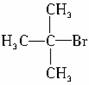
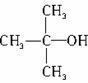
���п�ͼ��B��C��D������ط�Ӧ�е���Ҫ����(�����������Լ���ʡ��)���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ�
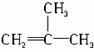
������ͼ�У�B�Ľṹ��ʽΪ________________������ȡ����Ӧ����____________(���ͼ�����)��������ȥ��Ӧ����___________(�����)��д����Ӧ�ܵĻ�ѧ����ʽ(ֻд��Ҫ���������Ӧ����)____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩ����� | ������� |
(CH3)2C=CHCH3 | 10.4 |
CH3CH=CH2 | 2.03 |
CH2= CH2 | 10.0 |
CH2=CHBr | 0.04 |
�ݱ������ݣ��ܽ�ϩ��������ʱ����Ӧ������C=C��ȡ���������ࡢ������Ĺ�ϵ��____________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������_________��
A.(CH3)2C=C(CH3)2
B.CH3CH=CHCH3
C.CH2=CH2
D.CH2=CHCI
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩ����� | ������� |
(CH3)2C=CHCH3 | 10.4 |
CH3CH=CH2 | 2.03 |
CH2=CH2 | 1.00 |
CH2=CHBr | 0.04 |
�ݱ������ݣ��ܽ�ϩ��������ʱ����Ӧ������C=C��ȡ���������ࡢ������Ĺ�ϵ��
��________________________________________________________________��
��________________________________________________________________��
��________________________________________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������______________(�����)��
A.(CH3)2C=C(CH3)2
B.CH3CH=CHCH3
C.CH2=CH2
D.CH2=CHCl
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com