
 ��ѧ��ѧ�г�������������ͼ��ʾ��ת����ϵ����Ӧ������ȥ����X��Y��Z��W�ǵ��ʣ������Ϊ�����A��W��Z�����³���̬����A��һ�ִ�����Ⱦ�B��һ�ֳ��õ��ᣮ
��ѧ��ѧ�г�������������ͼ��ʾ��ת����ϵ����Ӧ������ȥ����X��Y��Z��W�ǵ��ʣ������Ϊ�����A��W��Z�����³���̬����A��һ�ִ�����Ⱦ�B��һ�ֳ��õ��ᣮ
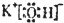 ���ʴ�Ϊ��
���ʴ�Ϊ��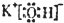 ��
��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ����Ӧ���������ɵ�ˮ��������������ȥ����
��ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E�����������NaOH��������ɷ������ֽⷴӦ����Ӧ���������ɵ�ˮ��������������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����A��E����ͬ��Ԫ�أ�������ѧ��ѧ�г��������ʣ����ǿɷ�����ͼ����ʾ�ķ�Ӧ����A��E���������������ȥ����
����A��E����ͬ��Ԫ�أ�������ѧ��ѧ�г��������ʣ����ǿɷ�����ͼ����ʾ�ķ�Ӧ����A��E���������������ȥ����| B | C | D | |
| ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com