
����Ҫ��������и�С��ʵ��Ŀ�ġ���a��bΪ���ɼУ����ȼ��̶�װ������ȥ��

(1)��֤̼����ǽ����Ե����ǿ��������֪���ԣ������� >̼�ᣩ
�ٽ�������_________________����ҩƷ��a�ر� b��Ȼ�����Ũ���ᣬ���ȡ�
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�ǣ� ��
װ��A�е��Լ������ǣ� ��
����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������ǣ� ��
(2)��֤ SO2�������ԡ���ԭ�Ժ������������ͨ�ԡ�
�ٴ�b���ر�a��
��H2S��Һ����dz��ɫ���dz��֣���ѧ����ʽ�ǣ�
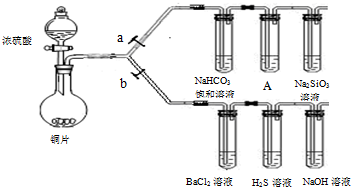
��BaCl2��Һ��������������ֳ����ݣ��քe�μ�������Һ���������ij����Ļ�ѧʽ�����±���Ӧλ�ã�

д��SO2����ˮ��Ӧ�����ӷ���ʽ ��
(1) �ټ��װ�������ԣ�2�֣� ��Cu + 2H2SO4(Ũ)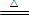 CuSO4 + SO2��+
2H2O��2�֣�
CuSO4 + SO2��+
2H2O��2�֣�
����KMnO4��Һ����ˮ��NaHCO3��Һ��2�֣� ��Na2SiO3��Һ�в�����״������2�֣�
(2) ��2H2S + SO2 = 3S��+ 2H2O��2�֣� ��BaSO4��2�֣� BaSO3��2�֣�
Cl2 + SO2 + 2H2O = SO42��+ 4H��+ 2Cl����2�֣�
����������1��װ��ͼ��װ���Ժ�����Ҫ����װ�õ������ԡ�Ũ�������ǿ�����ԣ��ڼ��ȵ��������ܰ�ͭ������������ͭ��SO2��ˮ�����������������ǿ��̼��ģ��������ɵ�SO2ͨ�뵽̼��������Һ�У�������CO2���塣��̼���������ǿ�ڹ���ģ�����CO2ͨ�뵽��������Һ�У����������ᾧ�塣Ϊ�˷�ֹSO2�Ĵ��ڸ���CO2�����Ƶķ�Ӧ����Ҫ��ͨ���������Һ֮ǰ���Ȱ�SO2��ȥ������ѡ�����Ը��������Һ����ˮ��̼��������Һ�ȡ�
��2��H2S�е���Ԫ�ش�����ͼۣ�SO2�ܰ������������ɵ�����ӦʽΪ2H2S + SO2 = 3S��+ 2H2O����������ǿ�����ԣ��ܰ�SO2�����������ᣬ�Ӷ��������ᱵ��������ˮ�������ᷴӦ����������泥��������������ᱵ������


 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | BaSO4 BaSO4 |
BaSO3 BaSO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�갲��ʡ��һ��ѧ�������ʼ컯ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ��̽����̽��̼����Ԫ�صķǽ����Ե����ǿ��
����Ҫ��������и�С��
��1��ʵ��װ�ã�

��д��ʾ��������A B
��2��ʵ�鲽�裺
����������_____________����ҩƷ��a��Ȼ�����Ũ���ᣬ����
��3������̽��������֪����ǿ��:������ >̼�ᣩ
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ;
װ��E����������KMnO4��Һ�������� ;
����˵��̼Ԫ�صķǽ����Աȹ�Ԫ�طǽ�����ǿ��ʵ�������� ;
�������Թ�D�е�ʵ�������ܷ�֤����Ԫ�صķǽ�����ǿ��̼Ԫ�صķǽ�����___����ܡ������Թ�D�з�����Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com