
| A�� | ��20g�����ƺ�17g�Ȼ��ط���100ml�ձ��У���35mLˮ�������ȡ����裬ʹ��ҺŨ����ԼΪԭ����һ��ʱ�����ȹ��˼��ɵõ�����ؾ��� | |
| B�� | ��ɫ��Ӧʵ���У���˿��պȡ������Һǰ��Ӧ����ϡ����ϴ�������� | |
| C�� | �к͵ζ�ʵ���У�������ʢ�Ŵ�����Һ����ƿϴ����δ�����Ӱ��ⶨ��� | |
| D�� | ��Һ��ȡҺ����Һ�ܴ�ֱ�����������棬�ɿ�ʳָʹ��Һȫ�������������ȡ����Һ�� |
���� A������ʱ������ص��ܽ������Ӧ��ȴ�ᾧ�õ�����أ�
B������ӷ�������ʵ�飻
C����ƿϴ����δ�����Ӱ�����Һ�����ʵ����ʵ�����
D����Һ��ȡҺ��Ӧ��ֱ��������б�������У��ܼ��������ڽӴ�������ʳָ��ʹҺ������������������ٵ�15�룬ȡ����Һ�ܣ�
��� �⣺A������ʱ������ص��ܽ�����������ܽ�����¶�Ӱ�첻ͬ������ȹ��˵õ�NaCl����ȴ�ᾧ�õ�����أ���A����
B������ӷ�������ʵ�飬Ӧѡ����ϴ�������գ���B����
C����ƿϴ����δ�����Ӱ�����Һ�����ʵ����ʵ�������Ӱ��ⶨ�������C��ȷ��
D����Һ��ȡҺ����Һ��������������Һ�������У�ʹ����ڼ�˽Ӵ����ڣ�ʹ������б����ʹ��Һ��ֱ����Ȼ���������ʳָ��ʹ��Һ���ɵ�˳�����£�����Һֹͣ������һ��ȴ�15�����ó�����D����
��ѡC��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰ʵ�������������ɫ��Ӧ���к͵ζ��ȣ��������ʵ����ʡ���Ӧԭ��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
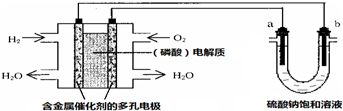
| A�� | ��ع���ʱ��������ӦʽΪ��O2+4H++4e-�T2H2O | |
| B�� | ���ʱ���������ݳ�amol���壬ͬʱ��W��Na2SO4�q10H2O �����������¶Ȳ��䣬ʣ����Һ�е������Ƶ�����������$\frac{71W}{161��W+36a��}$��100% | |
| C�� | ���ʱ����������·���ǣ����������·����������Һ������������ | |
| D�� | ����������ģ������������0.01g O2ʱ��b ����Χ�����0.02g H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 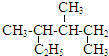 ��ϵͳ������3��4-�������� ��ϵͳ������3��4-�������� | |
| B�� | 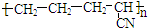 ���е���CH2=CH-CH3��CH2=CN�����Ӿ۷�Ӧ���õ��� ���е���CH2=CH-CH3��CH2=CN�����Ӿ۷�Ӧ���õ��� | |
| C�� | �������ļ�ȩ������[CH3CH��OH��COOH]��ȫȼ�����ĵ���������ȣ�������ˮ��������� | |
| D�� | ������Ӧ�� �����ڸ÷�Ӧ�����˲����ͻ������̼��˫������С���ӣ���˸÷�Ӧ������ȥ��Ӧ �����ڸ÷�Ӧ�����˲����ͻ������̼��˫������С���ӣ���˸÷�Ӧ������ȥ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
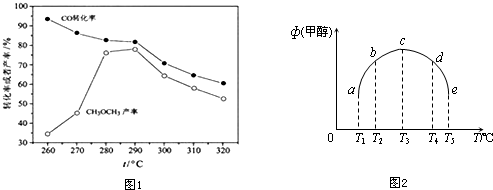
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
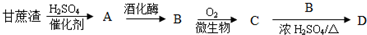
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
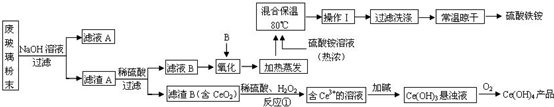

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
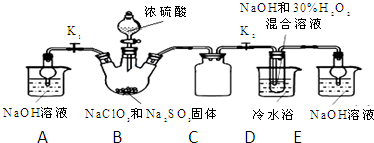
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ���� | ���� | |
| A | �ȵμ�BaCl2��Һ�ٵμ�HCl��Һ | ���ɰ�ɫ���� | ԭ��Һ����SO42- |
| B | �μ���ˮ��CCl4�������� | �ϲ���Һ�ԳȺ�ɫ | ԭ��Һ����Br- |
| C | �ýྻ��˿պȡ��Һ������ɫ��Ӧ | �������ɫ | ԭ��Һ����K+ |
| D | �μ�NaOH��Һ�����ȣ���ʪ���ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
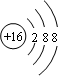 ��32g��������Ũ�����з�Ӧת�Ƶĵ�����Ϊ2NA��
��32g��������Ũ�����з�Ӧת�Ƶĵ�����Ϊ2NA���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com