
�Իش��������⣺
(1)�ȷ�������õ�(C2H4O)n�����������ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ��___________________��
(2)���һ��ʵ��֤�����õ���ȩ�Ƿ�����(д����Ҫ�������衢ʹ�õ��Լ���ʵ������ͽ���)��____________________________��
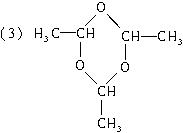
(3)��n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ_________________��
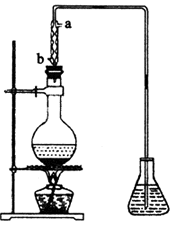
(4)��ȡ��ȩ��װ������ͼ����ƿ�е�Һ����(C2H4O)n��6 mol��L-1�Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ�С�
��������������ˮ�Ľ�����_________(�a����b��)��
����ʵ������в�ʹ����������ȴ�����������Ľ��У���Һ���к�ɫ���ʺʹ̼�����ζ�������ɡ����û�ѧ����ʽ��ʾ��һ����_____________________________________��
�۵���ƿ�ڵ��ܿ�����Խ��Խ��ʱ��������ȩ��������������ʵ����������ʵ��װ�õĵ�һ��������___________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�038
���õ���ȩˮ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�Ļ�״�ۺ��� �����ķе��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8�棬������Һ���ױ���������������ķе���117.9�棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ(�Եõ���ȩˮ��Һ)���練Ӧ�˴���ͼ������
�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8�棬������Һ���ױ���������������ķе���117.9�棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ(�Եõ���ȩˮ��Һ)���練Ӧ�˴���ͼ������
�Իش��������⣺
(1)�ȷ�������õ� ���䷽���ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ��________��
���䷽���ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ��________��
(2)���һ��ʵ��֤�����õ���ȩ�Ƿ�����(д����Ҫ�������衢ʹ�õ��Լ���ʵ������ͽ���)��________________________________________��
(3)��ȡ��ȩ��װ����ͼ��ʾ����ƿ�е�Һ���� ��
�� �Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�
�Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�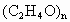 �����ֽ⣬���ɵ����嵼����ƿ�У�
�����ֽ⣬���ɵ����嵼����ƿ�У�
��������������ˮ�Ľ�����________(�a����b��)��
����ʵ������в�ʹ����������ȴ�����������Ľ��У���Һ���к�ɫ���ʺʹ̼�����ζ�������ɣ����û�ѧ����ʽ��ʾ��һ����________________________��
�۵���ƿ�ڵ��ܿ�����Խ��Խ��ʱ��������ȩ����������ʵ����������ʵ��װ�õĵ�һ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡʦ����2006��2007ѧ��ȸ����꼶�¿�(��)��ѧ���� ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���õ���ȩˮ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬��
��Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�Ļ�״�ۺ���(C2H4O)n�����ķе�
��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8��,������Һ���ױ�����,
��������ķе���117.9�棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ(�Եõ���ȩˮ
��Һ),���������·�Ӧ: (C2H4O)n ![]() nC2H4O��
nC2H4O��
�����Իش��������⣺
(1)�ȷ�������õ�(C2H4O)n�����������ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ��_________��
(2)���һ��ʵ��֤�����õ���ȩ�Ƿ�����(д����Ҫ��
�����衢ʹ�õ��Լ���ʵ������ͽ���)��______________��
�� (3)��n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ___________________________��
�� (4)��ȡ��ȩ��װ����ͼ����ƿ�е�Һ����(C2H4O)n��6mol/LH2SO4�Ļ�����ƿ��ʢ������ˮ�����Ȼ�
���������ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ�У�
����������������ˮ�Ľ�����________(�a����b��)��
���� ����ʵ������в�ʹ����������ȴ�����������Ľ��У���Һ���к�ɫ���ʺʹ̼�����ζ�������ɣ�
���û�ѧ����ʽ������һ����_________________________��
�۵���ƿ�ڵ��ܿ�����Խ��Խ��ʱ��������ȩ�Ѽ���ȫ��������ʵ����������ʵ��װ�õĵ�һ�������ǣ�____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���õ���ȩˮ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���� �ⶨ���ϲ�����Ϊ��ȩ�Ļ�״�ۺ���(C2H4O)n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8�棬������Һ���ױ���������������ķе���117.9�棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ���Եõ���ȩˮ��Һ�������������·�Ӧ��![]()
![]() n
n![]() ������
������
�Իش��������⣺
��1���ȷ�������õ�(C2H4O)n�����������ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ��____________��
��2�����һ��ʵ��֤�����õ���ȩ�Ƿ�������д����Ҫ�������衢ʹ�õ��Լ���ʵ������ͽ��ۣ���________________��
 ��3����n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ__________________��
��3����n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ__________________��
��4����ȡ��ȩ��װ����ͼ����ƿ�е�Һ����(C2H4O)n��6mol��LH2SO4 �Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�(C2H4O)n�ֽ⣬���ɵ����嵼����ƿ�У�
��������������ˮ�Ľ�����________���a����b������
����ʵ������в�ʹ����������ȴ�����������Ľ��У���Һ���к�ɫ���ʺʹ̼�����ζ�������ɣ����û�ѧ����ʽ��ʾ��һ����________________________��
�۵���ƿ�ڵ��ܿ�����Խ��Խ��ʱ��������ȩ��������������ʵ����������ʵ��װ�õĵ�һ�������ǣ�________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com