
�Ĵ�ʢ���屶�ӡ����屶��Ϊԭ�Ͽ��Ƶû�����A��A�Ľṹ��ʽ��ͼ��ʾ��
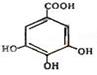
�������и��⣺
��1��A�ķ���ʽ�� ��
��2���л�������B������������¼��ȷ���������Ӧ�ɵõ�A��
��д��B�Ľṹ��ʽ��
��3����д��A�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��4���л�������C�Ǻϳ�����������ҩ���ԭ��֮һ��C���Կ�����B�����������ʵ���֮��1��2�����ӳɷ�Ӧ�õ��IJ��C���������ǻ���̼̼˫��ֱ�������Ľṹ����������ˮ��Ӧʹ��ˮ��ɫ��
��д��C����ˮ��Ӧ�Ļ�ѧ����ʽ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳ�P(һ�ֿ�������)��·�����£�
A(C4H10O)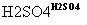 B(C4H8)
B(C4H8)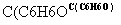
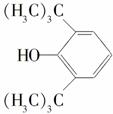
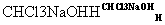
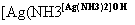 DE(C15H22O3)
DE(C15H22O3)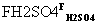 P(C19H30O3)
P(C19H30O3)
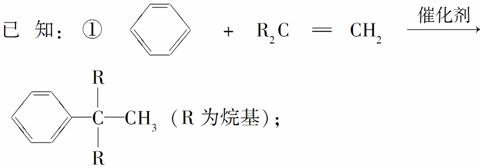
��A��F��Ϊͬ���칹�壬A����������������F������ֻ��һ������
(1)A��B�ķ�Ӧ����Ϊ________��B������������G(C4H10)��G�Ļ�ѧ������______________��
(2)A��ŨHBr��Һһ��������H��H�Ľṹ��ʽΪ______________��
(3)ʵ�����м���C��ѡ�������Լ��е�__________��
a�����ᡡ b��FeCl3��Һ�� c��NaHCO3��Һ�� d��Ũ��ˮ
(4)P������NaOH��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ________(�л����ýṹ��ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
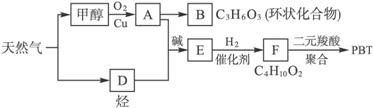
��Ȼ�������������е�֧����ҵ֮һ������Ȼ��Ϊԭ�Ͼ����з�Ӧ·�߿ɵù�������PBT��
��֪��
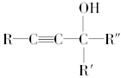 ��R��R�䡢R�塪������H��
��R��R�䡢R�塪������H��
��1��B���ӽṹ��ֻ��һ���⡢һ������һ��̼����B�Ľṹ��ʽ��_____________��B��ͬ���칹�����������Ǿ������ƽṹ����___________________����д�ṹ��ʽ��
��2��F�Ľṹ��ʽ��_____________��PBT����__________���л��߷��ӻ����
��3����A��D����E�ķ�Ӧ����ʽΪ____________���䷴Ӧ����Ϊ____________________��
��4��E��ͬ���칹��C���ܷ���������Ӧ����ʹ��ˮ��ɫ����ˮ���Ҳ����̼ԭ�������ȣ���G��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
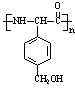
L��Hͨ���ļ����Ӷ��ɵĸ߾����L�Ľṹ��ʽΪ��
 ��
�� 
��2007�����Ͼ������л���ѧ������ģ�� (ѡ����)21.
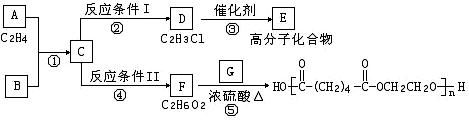
����ͼʾ�ش��������⣺
��1��д��A��E��G�Ľṹ��ʽ��A ��E ��G ��
��2����Ӧ�ڵĻ�ѧ����ʽ��������Ӧ�������� ��
��Ӧ�ܻ�ѧ����ʽ��������Ӧ�������� ��
��3��д���١��ݵķ�Ӧ���ͣ��� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
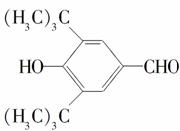
ij�л�������X��C7H8O������һ�л�������Y�������·�Ӧ���ɻ�����Z��C11H14O2����
X��Y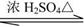 Z��H2O
Z��H2O
��1��X�����л�����֮һ����֪X������FeCl3��Һ������ɫ��Ӧ����X��________��������ĸ����
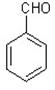
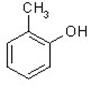

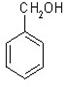 ��A�� ��B�� ��C�� ��D��
��A�� ��B�� ��C�� ��D��
��2��Y�ķ���ʽ��_____________�����ܵĽṹ��ʽ�ǣ�_____________��_____________��
��3��Y�ж���ͬ���칹�壬����һ��ͬ���칹��E����������Ӧ������ᆳ�ữ�ɵõ�F��C4H8O3����F�ɷ������·�Ӧ��F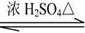
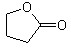 ��H2O
��H2O
�÷�Ӧ��������_____________��E�Ľṹ��ʽ��_____________��
��4����Y��E������ͬ��̼������Z�Ľṹ��ʽΪ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���Һ�Ⱥ���ˮ����������ȷ���ǣ���
A��Һ���Ǵ��������ˮ�ǻ����
B��Һ�������ԣ���ˮ������
C��Һ�Ƚ���ˮ��Ư�����ø�ǿ
D��Һ����ɫ����ˮ�ʻ���ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й������ʵı��治��ȷ���ǣ� ����
A��AgNO3��ҺӦ��������ɫƿ�� B����ˮ��������ɫϸ��ƿ��
C��Һ�ȿ��Ա����ڸ���ĸ�ƿ�� D��Ư�ۿ�¶���ڿ����б���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ܹ�ֱ�Ӽ���BaCl2��NaCl��Na2CO3������Һ���Լ���(����)
A��AgNO3��Һ B��ϡ����
C��ϡ���� D��ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ҫ�ɷ���CH4��0.5 mol CH4��ȫȼ������CO2��Һ̬ˮʱ���ų�445kJ�����������Ȼ�ѧ����ʽ�У���ȷ���ǣ��� ��
A��2CH4(g)+4O2(g)��2CO2(g)+4H2O(l) ��H=+890kJ/mol
B��CH4(g)+2O2(g)��CO2(g)+2H2O(l) ��H=+890kJ/mol
C��CH4(g)+2O2(g)��CO2(g)+2H2O(l) ��H=-890kJ/mol
D��1/2 CH4(g)+ O2(g)��1/2CO2(g)+ H2O(g) ��H=-890kJ/mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com