
��֪��A��B��C��D��E��F����Ԫ�صĺ˵�������������������ڱ���ǰ�����ڵ�Ԫ�ء�����Aԭ�ӵĺ�����3��δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ�������ߣ�F���γɺ�ɫ(��ש��ɫ)��F2O�ͺ�ɫ��FO���������
�ش��������⣺
(1)Fԭ�ӵ�M������Ų�ʽΪ________________��
(2)B��C��D�ĵ�һ��������С�����˳��Ϊ________________________________________________________________________
(��Ԫ�ط��ű�ʾ)��
(3)A�ļ��⻯����Ӽ�������ˮ������Ҫԭ����____________________________________��
(4)E�������������ӵĿռ乹����____________��������ԭ�ӵ��ӻ���ʽΪ__________��
(5)F�ĸ�������A�ļ��⻯���γɵ������ӣ���λ��Ϊ________��
(6)A��F�γɵ�ij�ֻ�����ľ����ṹ��ͼK348��ʾ�����仯ѧʽΪ________(��ɫ���ʾFԭ��)����֪���ڵİ��������֮��ľ���Ϊa cm����þ������ܶ�Ϊ__________g/cm3��

ͼK348
(1)3s23p63d10
(2)Na��Al��Si
(3)��������ˮ����֮��������
(4)ƽ���������Ρ�sp2
(5)4��(6)Cu3N����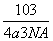 (����������Ҳ��)
(����������Ҳ��)
[����] Aԭ�ӵĺ�����3��δ�ɶԵ��ӣ�������Ų�ʽΪ1s22s22p3��ΪNԪ�أ�Eԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ������Ų�ʽΪ1s22s22p63s23p4��ӦΪSԪ�أ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�ΪAlԪ�أ�������B2E�ľ���Ϊ���Ӿ��壬BӦΪ�ڢ�A��Ԫ�أ���ԭ��������N��Al֮�䣬ӦΪNaԪ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�ӦΪSiԪ�أ����ʹ�Ϊԭ�Ӿ��壬�۵��ڵ�����������ߣ�F���γɺ�ɫ(��ש��ɫ)��F2O�ͺ�ɫ��FO���������ӦΪCuԪ�ء�
(1)Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��M������Ų�ʽΪ3s23p63d10��
(2)��Ԫ�����ڱ��У�ͬһ����Ԫ�صĵ�һ�����ܴ�����������ͬһ����Ԫ�صĵ�һ�����ܴ��ϵ�����С���ݴ˿��ж�����Ԫ�صĵ�һ�����ܵ�˳��ΪNa��Al��Si��
(3)A(N)�ļ��⻯������ǰ�����������������ˮ������Ҫԭ����N��O�ĵ縺��ǿ������֮�����γ������
(4)SO3�к���3���Ҽ����µ��Ӷ���Ϊ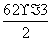 ��0�����Է��ӵĿռ乹����ƽ���������Σ�������ԭ�Ӳ���sp2�ӻ���
��0�����Է��ӵĿռ乹����ƽ���������Σ�������ԭ�Ӳ���sp2�ӻ���
(5)ͭ�����백������λ���γ������仯ѧʽΪ[Cu(NH3)4]2����
(6)���ݾ������������ķ��䷽�����㣬�����к���Nԭ�ӵ���ĿΪ8��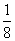 ��1��Cuԭ�ӵ���ĿΪ12��
��1��Cuԭ�ӵ���ĿΪ12��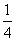 ��3�����仯ѧʽΪCu3N�����������֮��ľ���Ϊa cm���߳�Ϊ2a cm����
��3�����仯ѧʽΪCu3N�����������֮��ľ���Ϊa cm���߳�Ϊ2a cm����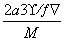 ��NA��1���������
��NA��1���������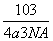 ��
��


 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС����ʵ���Һϳ���һ������A��
�ž�������A����Է�������������100��A��C��H�����������ֱ�Ϊ��
w(C)��69.76%��w(H)��11.63%������ȫȼ�պ����ֻ��CO2��H2O��
��A��Ħ������Ϊ __________ ��
�� A�ĺ˴Ź�����������ͼ��ʾ����A���Ժͽ����Ʒ�Ӧ����H2����������Cu�������±�������������ʾ���ǻ���̼̼˫�������Ľṹ���ȶ���
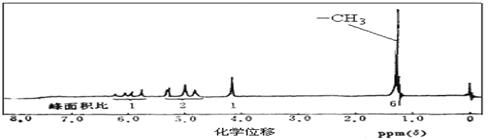
�����������Ϣд��A�Ľṹ��ʽ ��
(3)A��ij��ͬ���칹��B�����в���֧�����ܷ���������Ӧ��
��д��B����������Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����X���߶�BaSO4�������ϲ�����ԣ�ҽѧ���ڽ�������ϵͳ��X������ʱ������BaSO4���ڷ���Ӱ�������ּ���ֶγ�Ϊ�����ӡ�
(1)ҽѧ�Ͻ��б�����ʱΪʲô����BaCO3��(�����ӷ���ʽ��ʾ)________________________________________________________________________��
(2)ij����С��Ϊ��̽��BaSO4���ܽ�ȣ��ֱ�����BaSO4���룺
a��5 mL ˮ��
b��40 mL 0.2 mol/L ��Ba(OH)2��Һ��
c��20 mL 0.5 mol/L��Na2SO4 ��Һ��
d��40 mL 0.1 mol/L ��H2SO4 ��Һ�У��ܽ������͡�
�����ϸ���Һ�У�Ba2����Ũ���ɴ�С��˳��Ϊ________��
A��b��a��c��d B��b��a��d��c
C��a��d��c��b D��a��b��d��c
����֪298 Kʱ��Ksp(BaSO4)��1.1��10��10�����������£���Һb�е�SO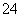 Ũ��Ϊ________ mol/L����Һc��Ba2����Ũ��Ϊ________ mol/L��
Ũ��Ϊ________ mol/L����Һc��Ba2����Ũ��Ϊ________ mol/L��
��ijͬѧȡͬ���������Һb����Һdֱ�ӻ�ϣ�����Һ��pHΪ________(������Һ�����Ϊ���ǰ����Һ�����֮��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��RΪǰ������Ԫ�أ���ԭ��������������XY2�Ǻ���ɫ���壻X����Ԫ�ؿ��γ�XH3��Z��̬ԭ�ӵ�M����K���������ȣ�R2����3d�������9�����ӡ�
��ش��������⣺
(1)Y��̬ԭ�ӵĵ����Ų�ʽ��________��Z���������е�һ��������������Ԫ����________��
(2)XY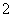 �����幹����________��R2����ˮ�������У��ṩ�µ��ӶԵ�ԭ����________��
�����幹����________��R2����ˮ�������У��ṩ�µ��ӶԵ�ԭ����________��
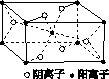
(3)Z��ijԪ���γɵĻ�����ľ�����ͼ��ʾ���������������������ӵĸ�������________��
(4)��R���ʵķ�ĩ����XH3��Ũ��Һ�У�ͨ��Y2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��W��R���ַ����ڲ�ͬ����Ķ�����Ԫ�أ�ԭ��������������X����̬�⻯�������ֻ��һ�Թµ��Ӷԣ�Y��Z��W������������Ӧ��ˮ��������������Ӧ��
(1)X�����ڱ��е�λ����________��Z3���ĺ�������Ų�ʽΪ________��
(2)Y��Z��R�ĵ�һ�����ܵĴ�С˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
(3)W���������������ķ��ӹ���Ϊ________����������������ˮ����������Һ���������е�W�ӻ��������Ϊ________��
(4)��R�ĵ�����Y������������Ӧ��ˮ�����ϣ��䷴Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(5)ͼK332��ʾΪY��Z�γɵĺϽ��ṹ���������1 mol Y�ĸúϽ�����������ˮ�г�ַ�Ӧ���ų���״������������Ϊ________L��

ͼK332
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���� ��ʾ�����й�����������������ȷ���ǣ� ��
��ʾ�����й�����������������ȷ���ǣ� ��
A. ���������� B. ����������
B. ����������
C. ���������� D. ������
D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶��£����淴ӦA(g)��3B(g) 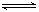 2C(g)�ﵽƽ��״̬�ı�־��
2C(g)�ﵽƽ��״̬�ı�־��
A. C���ɵ�������A�ֽ������2�����
B. ��λʱ������n mol A��ͬʱ����3n mol B
C. A��B��C��Ũ�Ȳ��ٱ仯
D. A��B��C�ķ�������Ϊ1��3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʷ��������ͨ������ɺ����ʡ��������ʷ����У�ֻ������ɵ���
A��Na2SO4����������������
B��HNO3��һԪ�ᡢǿ�ᡢ�ӷ�����
C��Mg(OH)2�Ƕ�Ԫ������Լ��ǿ��
D��Al2O3�����������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ʡҩƷ��ʱ�䣬�ס��ҡ�����λͬѧ��ͭƬ��пƬ��ϡ���ᡢCuSO4��Һ��ֱ����Դ��ʯī�缫�����ߡ��ձ����Թܵ���ѧ��ѧ������ҩƷ������(��Ʒ)��ͨ������Ĺ�˼������˱Ƚ�ͭ��п����� �����ǿ����ϵ��ʵ�顣����д���пհף�
�����ǿ����ϵ��ʵ�顣����д���пհף�
(1)��ͬѧ�ֱ�һСƬͭƬ��пƬ�����ձ��ײ�(ͭ��п���Ӵ�)�����ձ���С�ļ���ϡ���ᣬ�۲쵽��������________����ͬѧ�����˼·��_____________________________��
(2)��ͬѧ���ż�ʵ�飬���ձ��еμ�________��Һ�������۲쵽��������____________________________________________________________ ____________��
____________��
��ͬѧ����п��ͭ����������ǿ���Ľ��������ݵ�ԭ����____________________________________________________________________��
(3)��ͬѧʹ��ֱ����Դ��ʯī�缫��װ�õ��װ�ã�����ͬѧʵ������Һ�в����˱�Ҫ���Լ�________ ��Һ(��Ϊ���Һ)����Ӧ�ڵ������漴��ʼ��ʵ�����йط�Ӧ�Ļ�ѧ����ʽΪ________________________��ʵ���е�����������____ ____________________��
____________________��
(4)�����ٵ������һ����ʵ��(�Լ���������ѡ)��̽����֤ʵп��ͭ������Ե����ǿ��(��Ҫ˵������������)______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com