���й�ϵʽ����ȷ���ǣ� ��
A�������£�pH=2��CH3COOH��Һ��pH=12��NaOH��Һ�������Ϻ�c��CH3COOH��+c��H+����c��OH-��
B��1L0.3mol?L-1NaOH��Һ���ձ�״����4.48LCO2��c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+��
C��0.1mol?L-1��NH4Cl��Һ��0.05mol?L-1��NaOH��Һ�������Ϻ�c��Cl-����c��NH4-����c��Na+����c��OH-����c��H+��
D��0.1mol?L-1��Na2CO3��Һ�У�c��CO32-��+c��HCO3-��+c��H2CO3��=0.1mol?L-1
���𰸡�
������A������Ϊ���ᣬpH=2��CH
3COOH��Һ��pH=12��NaOH��Һ�������Ϻ����������Һ�����ԣ�
B.1L0.3mol?L
-1NaOH��Һ���ձ�״����4.48LCO
2��������3OH
-+2CO
2=CO
32-+HCO
3-+H
2O��CO
32-ˮ��̶ȴ���HCO
3-��
C.1mol?L
-1��NH
4Cl��Һ��0.05mol?L
-1��NaOH��Һ�������Ϻ���Һ�д���NH
4+��NH
3?H
2O����Һ�ʼ��ԣ�
D���������غ�ĽǶȷ�����
����⣺A������Ϊ���ᣬpH=2��CH
3COOH��Һ��pH=12��NaOH��Һ�������Ϻ����������Һ�����ԣ�
��c��H
+����c��OH
-��������һ������c��CH
3COOH��+c��H
+����c��OH
-������A��ȷ��
B��n��NaOH��=1L×0.3mol/L=0.3mol��n��CO
2��=
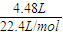
=0.2mol��
���߷�����3OH
-+2CO
2=CO
32-+HCO
3-+H
2O������CO
32-ˮ��̶ȴ���HCO
3-����CO
32-ˮ������HCO
3-����c��HCO
3-����c��CO
32-������B����
C.1mol?L
-1��NH
4Cl��Һ��0.05mol?L
-1��NaOH��Һ�������Ϻ���Һ�д���NH
4+��NH
3?H
2O����Һ�ʼ��ԣ�NH
3?H
2O����̶ȴ���ˮ��̶�NH
4+��
��c��Cl
-����c��NH
4-����c��Na
+����c��OH
-����c��H
+������C��ȷ��
D�����������غ㣬c��Na
2CO
3��=0.1mol/L����Na
2CO
3��CO
32-��HCO
3-��H
2CO
3���γɴ�������Һ�У���c��CO
32-��+c��HCO
3-��+c��H
2CO
3��=0.1mol?L
-1����D��ȷ��
��ѡB��
���������⿼������Ũ�ȵĴ�С�Ƚϣ���Ŀ�ѶȽϴ���ע��������ʵĵ���������ˮ���ԭ���İ��գ������״���ΪB��ע��CO
32-ˮ��̶ȴ���HCO
3-��

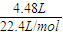 =0.2mol��
=0.2mol��

 �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�