
| 0.6mol |
| 2L |
| 1.6 mol |
| 2mol |
| ||
| 2min |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ
��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᣮ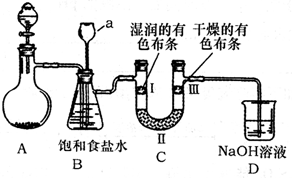
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��10 mL��Ͳȷ��ȡϡ������Һ8.0 mL
B.�ø����pH��ֽ�ⶨ��ˮ��pH
C.�ü�ʽ�ζ�����ȡKMnO4��Һ19.60 mL
D.ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��ƫ��
E.������FeCl3��Һ��������ˮ�м���Fe(OH)3����
F.Բ����ƿ����ƿ�����������ʱ��Ӧ����ʯ������
��2����50 mL 0.5 mol��L-1��������50 mL 0.55 mol��L-1��NaOH��Һ�����к��ȵIJⶨʵ�飬��ش��������⣺
����ʵ����ʹ�õ����������ձ���С�ձ������β������������ĭ���ϻ�ֽ������ĭ���ϰ��Ӳֽ�壨����������С�ף��⣬������Ҫ�õ���������___________________________��
��Ҫ������к��Ȳⶨ��ȷ�ԣ��ɲ��õĴ�ʩ�������г����������⣬������д�����֣�
a.������̲��еķ�������һ��ҪʹС�ձ���������ձ�������ƽ��
b.�����NaOH��ҺŨ�ȵ�����Ҫȷ����NaOH��Һ��Ũ�����Դ��������Ũ�ȡ�
c.ʵ�����ʱ����Ҫ�죬ע�ⲻҪ����Һ�������档
d.__________________________________________________________��
e.__________________________________________________________��
f.__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����������ʵ����ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��1�������й�ʵ���˵������������۲���ȷ���� ������ţ�
| A���Ʊ�Fe(OH)3����ʱ��Ӧ����ˮ�еμӱ���FeCl3��Һ�����������ȵ���Һ�ʺ��ɫΪֹ�� |
| B���ⶨ�к���ʵ���У�ÿ��ʵ���Ӧ���������¶ȣ���������ʼ�¶ȣ�NaOH��Һ����ʼ�¶Ⱥͷ�Ӧ����Һ������¶ȡ� |
| C����һ�����ʵ�����Һ���ƵĹ����У�û��ϴ���ձ��Ͳ�����������ʱ��ˮ�����˿̶��ߡ�����ƿû�и������ʹ������ҺŨ��ƫ�͡� |
| D����FeCl3��Һ�е���KI��������Һ����Һ����ɫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ������ԭƽһ�и߶����ϣ����л�ѧ�Ծ�����ͨ�ࣩ�������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com