



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ�����ֹ���ϲ���Һ��ɫ����ʧ |
| B��ʵ����������Һ�����ɫ��ֹͣ���ȣ�������ͨ����ϵʱ�ɲ��������ЧӦ |
| C������ˮ�Ҵ��м���ŨH2SO4��������170�����������ͨ������KMnO4��Һ����ɫ����ʧ |
| D���������Һ�м���ϡH2SO4�����ȼ����ӣ���ȴ���ٵμӼ�������Cu(OH)2��Һ�����ȣ��к�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

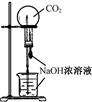
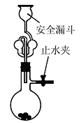
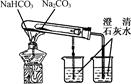
| A���ⶨһ��ʱ������,��H2�ķ�Ӧ���� | B����CO2��,��Ȫʵ�� | C��ʵ���������� | D���Ƚ�Na2CO3��,NaHCO3�����ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
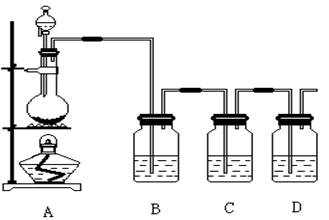
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
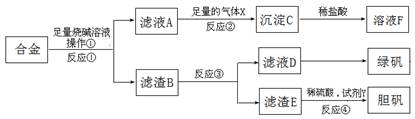
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������������Һ����һ����AgNO3��Һ���μӰ�ˮ������ǡ���ܽ⡣ |
| B���ڼ���ȩ������Cu(OH)2����Һʱ����һ����CuSO4��Һ�м�������NaOH��Һ |
| C����֤RXΪ����飬��RX���ռ�ˮ��Һ��ϼ��Ƚ���Һ��ȴ���ټ�����������Һ |
| D����ˮ�Ҵ���Ũ���Ṳ�ȵ�170�棬���Ƶõ�����ͨ�����Ը�����أ��ɼ����Ƶõ������Ƿ�Ϊ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com