
某同学取一定量的Al和Fe固体混合物,与2.0 L极稀的硝酸充分反应,反应过程中无气体放出。在反应结束后的溶液中,逐滴加入4 mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示。试回答下列问题: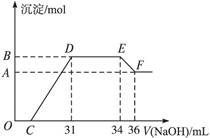
1、图中OC段没有沉淀生成,此阶段发生反应的离子方程式为 。
在DE段,沉淀的物质的量没有变化,则此阶段发生反应的离子方程式为 ;上述现象说明溶液中______________结合OH-的能力比___________强(填写离子符号)。
2、B与A的差值为______________mol。
3、B点对应的沉淀的物质的量为____________mol,C点对应的氢氧化钠溶液的体积为______________mL。
4、求原硝酸溶液的物质的量浓度?
 新思维假期作业寒假吉林大学出版社系列答案
新思维假期作业寒假吉林大学出版社系列答案科目:高中化学 来源: 题型:
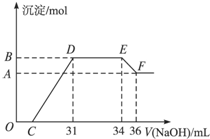 (2012?上海模拟)某同学取一定量的Al和Fe固体混合物,与2.0L极稀的硝酸充分反应,反应过程中无气体放出.在反应结束后的溶液中,逐滴加入4mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示.试回答下列问题:
(2012?上海模拟)某同学取一定量的Al和Fe固体混合物,与2.0L极稀的硝酸充分反应,反应过程中无气体放出.在反应结束后的溶液中,逐滴加入4mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示.试回答下列问题:查看答案和解析>>
科目:高中化学 来源: 题型:
某同学取一定量的Al和Fe固体混合物,与2.0 L极稀的硝酸充分反应,反应过程中无气体放出。在反应结束后的溶液中,逐滴加入4mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示。试回答下列问题:
1、图中OC段没有沉淀生成,此阶段发生反应的离子方程式为 。
在DE段,沉淀的物质的量没有变化,则此阶段发生反应的离子方程式为
;上述现象说明溶液中______________结合OH-的能力比___________强(填写离子符号)。
2、B与A的差值为______________mol。
3、B点对应的沉淀的物质的量为____________mol,C点对应的氢氧化钠溶液的体积为______________mL。
4、求原硝酸溶液的物质的量浓度?
查看答案和解析>>
科目:高中化学 来源:2011-2012学年上海市十校高三第二次联考化学试卷 题型:填空题
某同学取一定量的Al和Fe固体混合物,与2.0 L极稀的硝酸充分反应,反应过程中无气体放出。在反应结束后的溶液中,逐滴加入4 mol/L的氢氧化钠溶液,所加氢氧化钠溶液的体积(mL)与产生的沉淀的物质的量(mol)的关系如图所示。试回答下列问题:
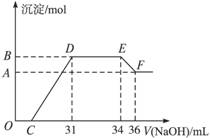
1、图中OC段没有沉淀生成,此阶段发生反应的离子方程式为 。
在DE段,沉淀的物质的量没有变化,则此阶段发生反应的离子方程式为
;上述现象说明溶液中______________结合OH-的能力比___________强(填写离子符号)。
2、B与A的差值为______________mol。
3、B点对应的沉淀的物质的量为____________mol,C点对应的氢氧化钠溶液的体积为______________mL。
4、求原硝酸溶液的物质的量浓度?
查看答案和解析>>
科目:高中化学 来源:上海模拟题 题型:计算题
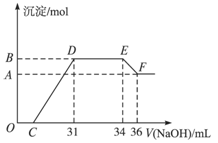
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com