
ЖўТШЛЏЖўСђ(S2Cl2)ЪЧЙуЗКгУгкЯ№НКЙЄвЕЕФСђЛЏМСЃЌЦфЗжзгНсЙЙШчгвЯТЭМЫљЪОЁЃГЃЮТЯТЃЌS2Cl2гіЫЎвзгыЫЎЗЂЩњЗДгІЃЌВЂВњЩњФмЪЙЦЗКьЭЪЩЋЕФЦјЬхЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ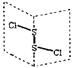
| AЃЎS2Cl2ЕФНсЙЙЪНЮЊClЃSЃSЃCl |
| BЃЎШєS2Br2гыS2Cl2НсЙЙЯрЫЦЃЌдђШлЗаЕуЃКS2Br2>S2Cl2 |
| CЃЎS2Cl2ЮЊКЌгаМЋадМќКЭЗЧМЋадМќЕФЗЧМЋадЗжзг |
| DЃЎS2Cl2гыH2OЗДгІЕФЛЏбЇЗНГЬЪНПЩФмЮЊЃК 2S2Cl2 + 2H2O Ёњ SO2Ёќ + 3SЁ§ + 4HCl |
C
НтЮіЪдЬтЗжЮіЃКИљОнФЃаЭПЩжЊЃЌЗжзгжаШЋВПЪЧЕЅМќЃЌAе§ШЗЃЛШєS2Br2гыS2Cl2НсЙЙЯрЫЦЃЌдђЯрЖдЗжзгжЪСПдНДѓЃЌШлЗаЕудНИпЃЌМДШлЗаЕуЪЧS2Br2>S2Cl2ЃЌBе§ШЗЃЛCВЛе§ШЗЃЌИУЛЏКЯЮяВЛЪЧЗЧМЋадЗжзгЃЌЖјЪЧМЋадЗжзгЃЛS2Cl2гіЫЎвзгыЫЎЗЂЩњЗДгІЃЌВЂВњЩњФмЪЙЦЗКьЭЪЩЋЕФЦјЬхЃЌдђЩњГЩЮягІИУЪЧSO2ЃЌдђDе§ШЗЃЌД№АИбЁCЁЃ
ПМЕуЃКПМВщЖўТШЛЏЖўСђНсЙЙЁЂаджЪЕФХаЖЯ
ЕуЦРЃКИУЬтЪЧИпПМжаЕФГЃМћПМЕуКЭЬтаЭЃЌЪєгкжаЕШФбЖШЪдЬтЕФПМВщЃЌЪдЬтЛљДЁадЧПЃЌВржиЖдбЇЩњЛљДЁжЊЪЖЕФЙЎЙЬКЭбЕСЗЃЌжМдкПМВщбЇЩњСщЛюдЫгУЛљДЁжЊЪЖНтОіЪЕМЪЮЪЬтЕФФмСІЁЃИУЬтЕФЙиМќЪЧУїШЗЛЏКЯЮяЕФНсЙЙЬиЕуЃЌШЛКѓНсКЯЬтвтСщЛюдЫгУМДПЩЃЌгажњгкХрбјбЇЩњЕФТпМЭЦРэФмСІЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЖўТШЛЏЖўСђЃЈS2Cl2ЃЉЪЧЙуЗКгУгкЯ№НКЙЄвЕЕФСђЛЏМСЁЃвбжЊЫќЕФНсЙЙЪНЮЊClЈDSЈDSЈDClЃЌвзгыЫЎЗДгІЃК2S2Cl2+2H2O 4HCl+SO2Ёќ+2SЁ§ЁЃЖдИУЗДгІЯТСаЫЕЗЈе§ШЗЕФЪЧ ЃЈ ЃЉ
AЃЎS2Cl2МШзїбѕЛЏМСгжзїЛЙдМС
BЃЎS2Cl2жЛзїбѕЛЏМС
CЃЎУПЩњГЩ1mol SO2зЊвЦ4molЕчзг
DЃЎS2Cl2жЛзїЛЙдМС
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
(08дцзЏШ§ея)ЖўТШЛЏЖўСђЃЈS2Cl2ЃЉЪЧЙуЗКгУгкЯ№НКЙЄвЕЕФСђЛЏМСЁЃвбжЊЫќЕФНсЙЙЪНЮЊClЈDSЈDSЈDClЃЌвзгыЫЎЗДгІЃК2S2Cl2+2H2O 4HCl+SO2Ёќ+2SЁ§ЁЃЖдИУЗДгІЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎS2Cl2МШзїбѕЛЏМСгжзїЛЙдМС
BЃЎS2Cl2жЛзїбѕЛЏМС
CЃЎУПЩњГЩ1mol SO2зЊвЦ4molЕчзг
DЃЎS2Cl2жЛзїЛЙдМС
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com