
| Ӧ�����������/mL | Ӧѡ������ ƿ�Ĺ��/mL | ������ƿ�� ����Ҫ���������� |
| | | |
| A��û�н���ϴ���ձ��� | B��������ˮʱ���������˿̶ȣ� | C��������ʱ���ӿ̶��ߣ� | D����ȡŨ����ʱ������Ͳ��E������Ͳϴ�Ӳ���ϴ��Һת�Ƶ�����ƿ�У�F��ת����Һǰ����ƿ��������ˮ�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��û�н�ϴ��Һת�Ƶ�����ƿ�� |
| B������ƿϴ����û�����ﴦ�� |
| C��ת�ƹ�������������Һ���� |
| D����ˮʱ��ˮ���������˿̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
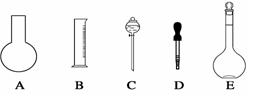
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �������ƶ���ȷ����
�������ƶ���ȷ����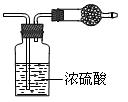
| A��������ڹ���Ϊ��ʯ�� | B��ԭ������һ����NO��O2 |
| C��ԭ������һ����NH3��NO��CO2 | D��ԭ������һ��û��HCl��Br2��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��294.7 L | B��378.0 L | C��2240.0 L | D��1120.0 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��װ�âٳ����ڷ��뻥�����ܵ�Һ�� | B��װ�âڿ���������NH3 |
| C��װ�âۿ������Ʊ��������� | D��װ�âܿ������ռ�NO��CO2������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com