
 3��Һ���ش���������
3��Һ���ش��������� CO3����������Һ��������ƿ���Ҫ����������
CO3����������Һ��������ƿ���Ҫ����������
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���ữ��BaCl2��Һ��ֻ�۲쵽�а�ɫ�������ɡ�
���ữ��BaCl2��Һ��ֻ�۲쵽�а�ɫ�������ɡ� ���������Ĺ�ϵ��ͼ��ʾ��
���������Ĺ�ϵ��ͼ��ʾ�� ���ʣ�
���ʣ�| �� �� | ȼ���ȣ�kJ��mol��1�� |
| H2��g�� | ��285��8 |
| CO��g�� | ��283��0 |
| CH4��g�� | ��890��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ýᾧ����ˮ��Һ�л���Ȼ������� |
| B����ֽ�ϲ����������Ȼ�����Һ���Ƿ���������Ȼ�ͭ |
| C��Ϊȷ�ⶨ������NaOH��Һ��Ӧ���к��ȣ�������ͼ�����ʵ���������� |
| D����AgNO3��Һ��ϡ��������������ƺ�ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A��ͭ��Ũ���ᷴӦ��NO2 |
| B��ͭ��ϡ���ᷴӦ��NO |
| C���Ҵ���Ũ���ᷴӦ����ϩ |
| D��ʯ��ʯ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���٢ܢۢ� | B���٢ۢܢ� | C���ܢ٢ۢ� | D���ܢ٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���ٷ�ֹ�������� |
| B���ڹ���ͭпԭ��� |
| C���۹���ͭ��ԭ��� |
| D������֤NaCl��Һ(����̪)������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
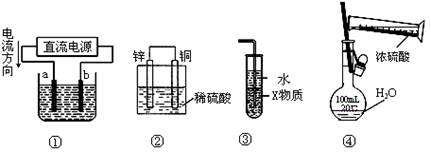
| A��������װ�âپ���ͭ����a��Ϊ��ͭ��b��Ϊ��ͭ���������ҺΪCuSO4��Һ |
| B��װ�â���ԭ��أ�����ʱSO42��������ͭ���ƶ� |
| C��װ�â���X��Ϊ���Ȼ�̼�����װ�ÿ��������հ���������ֹ���� |
| D��װ�âܿ�����ʵ��������һ�����ʵ���Ũ�ȵ�ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��
�� _____��
_____��| A����ѧ������ȡŨ����ʱ�����ӿ̶��� |
| B����������NaOHʱ�����������Ʒ��λ�õߵ���û��ʹ�����룩 |
C���ܽ�H2SO4����ʱû����ȴ�����¾�������ɺ�������Ʋ� ���� ���� |
D�����ձ����ܽ� ����ʱ������������Һ ����ʱ������������Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com