��2010?����ģ�⣩������أ�K
2FeO
4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl
2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�
��1����������ǿ�����Һ�м����������η�����Ӧ�����ӷ���ʽ��
��Fe
3++3OH
-=Fe��OH������
2Fe��OH��3+3ClO-+10OH-=2FeO2-4+3Cl-+5H2O
2Fe��OH��3+3ClO-+10OH-=2FeO2-4+3Cl-+5H2O
��
��2�������������ˮ���ͷŴ�����ԭ�������Ӷ��dz���Ч��ɱ��ˮ�еIJ����Ͳ��������ͬʱ����������ԭ������̬��Fe��OH��
3������һ��Ʒ�������������������ܸ�Ч�س�ȥˮ�е�ϸ�����������K
2Fe
2O
4��Һ��pH=4.74����Һ�У����Ƴ�c��FeO
2-4��=1.0mmol?L
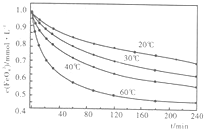
-1�������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc��FeO+2-
4���ı仯�������ͼ�����������ˮ��Ӧ�����ӷ�Ӧ����ʽΪ
4FeO2-4+10H2O=4Fe��OH��3+8OH-+3O2��
4FeO2-4+10H2O=4Fe��OH��3+8OH-+3O2��
���÷�Ӧ�ġ�H
��
��
0���������������=������
��3���������λ���һ��������ܵ�صIJ��ϣ��������ɵĵ�������ߣ��ŵ�������ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K
2FeO
4+8H
2O
3Zn��OH��
3+2Fe��OH��
3+4KOH�õ�طŵ�ʱ�ĸ�����ӦʽΪ
Zn+2OH--2e-=Zn��OH��
Zn+2OH--2e-=Zn��OH��
�������·��5.418��10
22������ͨ������������
5.94
5.94
g������ز��뷴Ӧ��
��4���ⶨijK
2FeO
4��ҺŨ�ȵ�ʵ�鲽�����£�
����1��ȷ��ȡV mL K
2FeO
4��Һ���뵽��ƿ��
����2����ǿ������Һ�У��ù���CrO
-2��FeO
2-4��Ӧ����Fe��OH��
3��CrO
2-4����3��������ϡ���ᣬʹCrO
2-4ת��ΪCr
2O
2-7��CrO
-2ת��ΪCr
3+��Fe��OH��
3ת��ΪFe
2+����4�������������������ָʾ������c mol?L
-1��NH
4��
2Fe��SO
4��
2����Һ�ζ����յ㣬���ģ�NH
4��
2Fe��SO
4��
2��ҺV
1mL��
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ
6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O
6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O
��
��ԭ��Һ��K
2FeO
4��Ũ��Ϊ
���ú���ĸ�Ĵ���ʽ��ʾ����

 ��2010?����ģ�⣩������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�
��2010?����ģ�⣩������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
 ��2010?����ģ�⣩ά����C��������Ѫ�ᣬ�������Ժ�ǿ��ԭ�ԣ�Ҳ��һ�ֳ�����ʳƷ�����ϵ����Ӽ�����ƻ��֭�ȣ�����ṹ��ͼ�������й�˵����ȷ���ǣ�������
��2010?����ģ�⣩ά����C��������Ѫ�ᣬ�������Ժ�ǿ��ԭ�ԣ�Ҳ��һ�ֳ�����ʳƷ�����ϵ����Ӽ�����ƻ��֭�ȣ�����ṹ��ͼ�������й�˵����ȷ���ǣ�������