
�������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ���㷺Ӧ���ڻ���ɱ��������������
��������ƾ�����Ʊ�
��ҵ������CaO2��8H2O����Ҫ�������£�
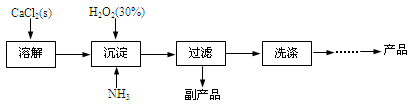
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2������ʱ���ñ�ˮ�����¶���10�����º�ͨ�������NH3�������ԭ��ֱ���
�� ���� ��
��������ƾ��庬���IJⶨ
ȷ��ȡ0.3000g��Ʒ����ƿ�У�����30 mL����ˮ��10 mL 2.000 mol��L��1 H2SO4����0.0200 mol��L��1KMnO4����Һ�ζ����յ㡣�ظ������������Ρ�H2O2��KMnO4��Ӧ�����ӷ���ʽΪ2MnO4����5 H2O2��6H+ ��2Mn2+��5O2����8H2O
��3���ζ��յ�۲쵽������Ϊ ��
��4�����ݱ�1���ݣ������Ʒ��CaO2��8H2O������������д��������̣�
|
����� |
��Ʒ������/g |
KMnO4��Һ�����/mL |
|
|
�ζ�ǰ�̶�/mL |
�ζ���̶�/mL |
||
|
1 |
0.3000 |
1.02 |
24.04 |
|
2 |
0.3000 |
2.00 |
25.03 |
|
3 |
0.3000 |
0.20 |
23.24 |
��1. KMnO4����Һ�ζ�����
18����12�֣�
��1��CaCl2+H2O2+2NH3+8H2O��CaO2��8H2O��+2NH4Cl ��2�֣�
��2�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ����ֹ��������ķֽ⣩��2�֣�
��ͨ�����NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣨��ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⣬����߲�Ʒ�IJ��ʡ���˼��������֣���2�֣�
��3�����������һ��KMnO4����Һ����Һ����ɫ��dz��ɫ����30s����ɫ��2�֣�
��4��82.91%
5(CaO2��8H2O)��5H2O2��2KMnO4��1�֣�
n(CaO2��8H2O)��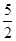 n(KMnO4)
n(KMnO4)
��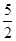 ��0.0200 mol��L��1��23.03mL��10��3L��mL��1��1�֣�
��0.0200 mol��L��1��23.03mL��10��3L��mL��1��1�֣�
��1.151��10��3 mol��1�֣�
CaO2��8H2O����������Ϊ�� ��100%��82.91%��1�֣�
��100%��82.91%��1�֣�
��������
�����������1��д��ѧ����ʽҪ�ӷ�Ӧ������������֣���ȷ���Ƿ���������ԭ��Ӧ���������ǿ�����Ӧ����CaCl2��H2O2��NH3��������CaO2��8H2O�����������غ㶨�ɿ�֪��һ��������NH4Cl���������Ӧ��Ԫ�ػ��ϼ�û�з����仯�����Բ���������ԭ��Ӧ����д��CaCl2+H2O2+NH3����CaO2��8H2O��+NH4Cl���ٸ��ݹ۲취������ƽ�����ȷ����Ӧ�ﻹ��Ҫˮ�μӷ�Ӧ��
��2����Ŀ�ṩ�������DZ�ˮ�����¶���10�����º�ͨ�������NH3�����������뵽���Ǽ��ٹ�������ķֽ⣬��һ��ԭ����������Ŀ����������Ϣ��������ƾ���[CaO2��8H2O]���ȶ����ʰ�ɫ������ˮ��������������Һ�����ͨ�����NH3ʹ��Һ�ʼ��ԣ�����CaO2��8H2O���ܽ⡣
��3���������ζ�ԭ�����������ָʾ����ɫ��ȷ���ζ��յ㣬������������KMnO4������H2O2����Ϊ�Ϻ�ɫKMnO4��Һ�����������ᱻ��ԭ����ɫ��Mn2+�����Բ���Ҫ�������ָʾ����ȷ���ζ��յ㣬���������һ��KMnO4����Һ����Һ����ɫ��dz��ɫ����30s����ɫʱ�������϶�����ζ��յ㡣
��4���ಽ��Ӧ����������ǻ�ѧ��Ӧԭ���ж����Ӧ������������ʼ����Ŀ����֮�����ȷ�������Ĺ�ϵ������ʱӦ��д���йط�Ӧ�Ļ�ѧ����ʽ���ϵʽ�����ݷ���ʽ�ҳ�������Ӧ�Ĺ����в�ͬ��Ӧ����֮�䷴Ӧ����������ʵ����Ĺ�ϵ�����ȷ����֪���Ŀ�����֮������ʵ����Ĺ�ϵ���г�����ʽ��⣬�Ӷ���������̡�
���ݱ���1���ṩ�����ݣ�����Ҫ������εζ����ĵ�KMnO4��ƽ��ֵΪ23.03mL��
2MnO4����5 H2O2��6H+ ��2Mn2+��5O2����8H2O
CaCl2+H2O2+2NH3+8H2O��CaO2��8H2O��+2NH4Cl
�ٸ�������������ʽ�ó�MnO4����CaO2��8H2O�Ĺ�ϵ��
n(CaO2��8H2O)��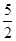 n(KMnO4)
n(KMnO4)
��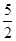 ��0.0200 mol��L��1��23.03mL��10��3L��mL��1��1�֣�
��0.0200 mol��L��1��23.03mL��10��3L��mL��1��1�֣�
��1.151��10��3 mol��1�֣�
CaO2��8H2O������������ ��100%��82.91%
��100%��82.91%
���㣺�����Թ�������Ϊ��������Ԫ�ؼ����������ʡ�����к͵ζ�ԭ�����ಽ�軯ѧ��������֪ʶ��


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08������ģ��)����������һ�ְ�ȫ�������ʣ��������������ȵĽᾧˮ�����ڳ�ʱ����ã�ͨ�������в���CaO��Ϊ�ⶨ��Ʒ�й������Ƶ�����뺬������������ʵ�飺
��1����ȡ0.542g����������Ʒ������ʱ�������·�Ӧ��
2CaO2?XH2O![]() 2CaO��O2����2XH2O���ڱ�״���µõ���O2���Ϊ67.2mL�������Ʒ��CaO2�����ʵ���Ϊ ��
2CaO��O2����2XH2O���ڱ�״���µõ���O2���Ϊ67.2mL�������Ʒ��CaO2�����ʵ���Ϊ ��
��2����ȡͬһ��Ʒ0.542g������������ϡ�����У�Ȼ�����������Na2CO3��Һ������Һ��Ca2��ȫ��ת��ΪCaCO3�������õ������CaCO3 0.700g��
�ټ�����Ʒ��CaO2?XH2O��Xֵ��
�����û�ѧʽ��ʾ����Ʒ����ɣ�Ӧ��ʾΪ ��
��3���������Ʒ�й������ƾ���Ĵ����Ƕ��٣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com