
 ʵ����һ�Լ�ƿ�ı�ǩ��������ֻ����Լ������ͼһ���֣�
ʵ����һ�Լ�ƿ�ı�ǩ��������ֻ����Լ������ͼһ���֣�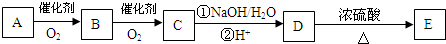

 ��
�� ��
��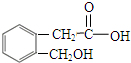 ��
��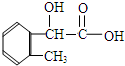 ��
�� ��
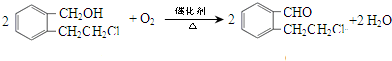
�� ��Ũ���������·���ȡ����Ӧ����E��EΪ
��Ũ���������·���ȡ����Ӧ����E��EΪ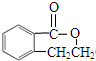 ��C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ���ټ�����������Һ�����ְ�ɫ������C�к�����ԭ�ӣ�C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ��õ�
��C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ���ټ�����������Һ�����ְ�ɫ������C�к�����ԭ�ӣ�C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ��õ� ����CΪ
����CΪ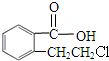 ��B�������õ�
��B�������õ� ��BΪ
��BΪ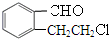 ��A�������õ�
��A�������õ�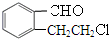 ��AΪ
��AΪ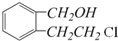 ���ٽ�϶�Ӧ�л���Ľṹ�����ʣ��Լ�ǰ��ת����ϵ�������⣮
���ٽ�϶�Ӧ�л���Ľṹ�����ʣ��Լ�ǰ��ת����ϵ�������⣮ ��
�� ��
��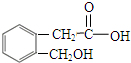 ��
�� ����4�֣�
����4�֣� ��
�� ��4��
��4�� ��C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ���ټ�����������Һ�����ְ�ɫ������C�к�����ԭ�ӣ�C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ��õ�
��C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ���ټ�����������Һ�����ְ�ɫ������C�к�����ԭ�ӣ�C��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ��õ� ����CΪ
����CΪ ��B�������õ�
��B�������õ� ��BΪ
��BΪ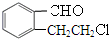 ��A�������õ�
��A�������õ�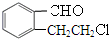 ��AΪ
��AΪ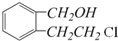 ��
��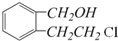 ��
�� ��
�� ��
�� ��Ũ���������·���ȡ����Ӧ����E��EΪ
��Ũ���������·���ȡ����Ӧ����E��EΪ ����Ӧ����ʽΪ��
����Ӧ����ʽΪ��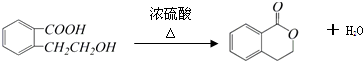 ��
�� ��
��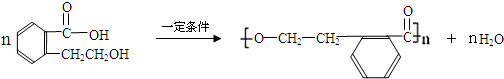 ��
��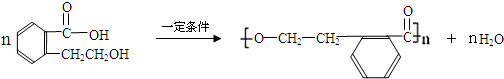 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� ����ʽ��HCl ��Է���������36.5 �ܶȣ�1.19g/mL HCl������������36.5%��1����Ũ������HCl�����ʵ���Ũ��Ϊ 11.9mol/L 11.9mol/L ����2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯���� BD BD �����ţ���A����Һ��HCl�����ʵ���B����Һ��Ũ��C����Һ��Cl-����ĿD����Һ���ܶ� ��3��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.400mol/L��ϡ���ᣮ�ø�ѧ����Ҫ��ȡ 16.8 16.8 mL��С�������һλ������Ũ����������ƣ�������a��10mL��Ͳ��b��25mL��Ͳ��c���ձ���d��������ƽ��e.500mL����ƿ��f����ͷ�ιܣ�g�����������������ѡȡ��Ҫ������������������һ��ʹ���Ⱥ�˳������ bcgef��bfcge bcgef��bfcge �����ţ����������ƹ����У�����ʵ��������������������ʵ���Ũ���к�Ӱ�죿���ڿո����ƫ�ߡ�����ƫ�͡�����Ӱ�족��������ʱ���ӹ۲� ƫ�� ƫ�� �����ݺ���ҡ�ȡ����ú���Һ���½����ټ�����������ˮƫ�� ƫ�� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ��ɽ�صڶ���ѧ��һ��һ���¿���ѧ�Ծ����������� ���ͣ�ʵ���� (12��)����5����������ʵ�鼼�ܿ��飬ij��ȤС���ͬѧ�ڰ�����ʦ����ѧʵ��ʱ������ʵ��̨������ڷŵ�ҩƷ�У�����ͼ������һ�Լ�ƿ�ı�ǩ����Ϊ�˼������Լ��ɷ֣�ͬѧ����������̽����
SiO2������Ӧ���仯ѧ����ʽΪ2NaOH + SiO2=" X" + H2O������Xʹƿ��������ճ����һ�� ���Ʋ�X�Ļ�ѧʽΪ_________________�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ��һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ���� (12��)����5����������ʵ�鼼�ܿ��飬ij��ȤС���ͬѧ�ڰ�����ʦ����ѧʵ��ʱ������ʵ��̨������ڷŵ�ҩƷ�У�����ͼ������һ�Լ�ƿ�ı�ǩ����Ϊ�˼������Լ��ɷ֣�ͬѧ����������̽���� ��������⡿��ƿ�Լ��ijɷ���ʲô��
[����]���������ǩ��ʵ����ҩƷ����ڷŵ�ԭ����ƿ�Լ����ܲ���_______________________�� A���� B���� C���� [����]�ٿ�����NaOH��Һ���ڿ�����Na2CO3��Һ�� �ۿ�����_______________________��дһ�֣��� [̽���] ��1��С��ȡ����������С�Թ��У��μӷ�̪��Һ����Һ��Ϊ��ɫ���ɴ�С����Ϊ�������ȷ�� ��ͬѧ�ǽ������ۺ�һ����ΪС���ķ��������У�������_______________________ ��2��С��ͬѧ��Ϊ����ȻС����ʵ�鲻�ܵõ���ȷ�Ľ��ۣ���ֻҪ������Һ�еμ�һ���Ȼ� ����Һ��ͬ���ܵó���ȷ�Ľ��ۡ�
��3��С������Ҫ��һ��ȷ����1���еijɷ֣�ֻ��ѡ����ͼ���е��Լ����ܰ��������������������ǽ���������ʵ�顣
[ʵ�鷴˼]ʵ����ʢ��NaOH��Һ���Լ�ƿ�����ò���������ԭ���ǣ�NaOH�벣���е� SiO2������Ӧ���仯ѧ����ʽΪ2NaOH + SiO2= X + H2O������Xʹƿ��������ճ����һ�� ���Ʋ�X�Ļ�ѧʽΪ_________________��
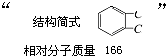
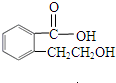
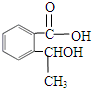
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��0108 ������ ���ͣ������ ʵ����һҩ��ƿ�ı�ǩ��������ֻ������ԼԼ��������һ����: �����л���Ľṹ��ʽ��  �����ṹ��ʽ�Ҳ����𣩡���Է�������166�� ȡ��ҩ��ƿ�е��Լ���ͨ��ȼ��ʵ���ã�16.6g��������ȫȼ�յõ�39.6gCO2��9g H2O�� �����ṹ��ʽ�Ҳ����𣩡���Է�������166�� ȡ��ҩ��ƿ�е��Լ���ͨ��ȼ��ʵ���ã�16.6g��������ȫȼ�յõ�39.6gCO2��9g H2O����1��ͨ�������֪���л���ķ���ʽΪ_________________ �� ��2����һ��ʵ���֪���ٸ������ܺ�̼��������Һ��Ӧ������ɫ��ζ���塣��16.6g�����������������Ʒ�Ӧ����������2.24L(��״��)��������ʵĽṹ������______________ ������� ��3��������ת���е�D��(2)�п��ܽṹ�е�һ�֣��ҿɷ�����ȥ��Ӧ��E��������Ԫ����ȡC��NaOHˮ��Һ�з�Ӧ��Ļ��Һ���������������ữ����������Һ�����ְ�ɫ������  ��A�Ľṹ��ʽΪ__________________ �� �ڷ�Ӧ���ͣ�A ��B__________________________ �� C��D__________________________ �� ��д����D��ȡE�Ļ�ѧ����ʽ________________________________________ �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |