
�һ����ȩ(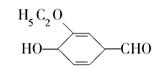 )��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ����Ũ����
)��ʳƷ���Ӽ�������ԭ�ϣ�����ζ�����ȩ����Ũ����
(1)�һ����ȩ�����еĺ������������������� (д����)�� (д����)���˴Ź����ױ����÷������� �ֲ�ͬ���͵���ԭ�ӡ�
(2)�һ����ȩ��ͬ���칹��A��һ���л��ᣬA�ɷ������±仯��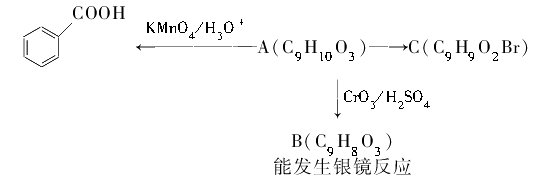 ��ʾ��
��ʾ�� b���뱽��ֱ��������̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ�
b���뱽��ֱ��������̼ԭ��������ʱ����̼ԭ�Ӳſɱ�����KMnO4��Һ����Ϊ�Ȼ�
��д��A�Ľṹ��ʽ�� ��
��д���ڼ���������C��NaOHˮ��Һ������Ӧ�Ļ�ѧ����ʽ�� ��
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A��B��C��D��E��F��G��H�ת����ϵ����ͼ��ʾ��5.2 g F����100 mL 1 mol/L NaOH��Һǡ����ȫ�кͣ�0.1 mol F����������NaHCO3��Ӧ���ڱ�״���·ų�4.48 L CO2��D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���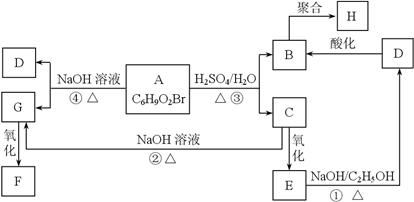
��1��д������C�еĹ����ŵ����ƣ� ��
��2��д������F��H�Ľṹ��ʽ��
F ��H ��
��3��д����Ӧ�١��ܵĻ�ѧ��Ӧ���ͣ��� ���� ��
��4��д���仯�١��۵Ļ�ѧ����ʽ��
��
��
��5��д����Է���������B��14������B������ͬ�����ŵ��������ʵĽṹʽ�������������칹����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18�֣�����2.8g�л���A����ȫȼ������0.15molCO2��1.8gH2O��A������ͼ����ͼ��ʾ����֪��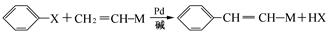 ��XΪ±ԭ�ӣ�MΪ������������ȡ������)�����л���A�ϳ�G���㶹�أ��IJ������£�
��XΪ±ԭ�ӣ�MΪ������������ȡ������)�����л���A�ϳ�G���㶹�أ��IJ������£�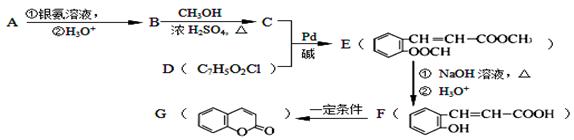
�ش��������⣺
��1��A�ķ���ʽΪ ��
��2��д��C�к�������������:�� ����F��G �ķ�Ӧ������ ��
��3��д��A��������Һ��Ӧ�Ļ�ѧ����ʽ�� ����
��4��D�Ľṹ��ʽΪ�� ����
��5�������㶹�أ�  ���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨
���������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨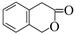 ����Ҫ�õ����Լ��У�NaOH��Һ���� ����
����Ҫ�õ����Լ��У�NaOH��Һ���� ����
��6��F�ж���ͬ���칹�壬д��ͬʱ������������������ͬ���칹��Ľṹ��ʽ�� ����
��. �����г������⣬��������״�ṹ�� ��.���������������ڶ�λ��ȡ������
��. �ܷ���ˮ�ⷴӦ��������Na��Ӧ�� ��.��������Cu(OH)2�����ʵ�����1:2����������Ӧ
��7����֪��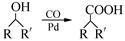 (R��R��Ϊ����)����д���Ա��ͱ�ϩ��
(R��R��Ϊ����)����д���Ա��ͱ�ϩ��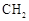 ��CH��CH3��Ϊԭ�ϣ��ϳ�
��CH��CH3��Ϊԭ�ϣ��ϳ�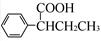 ��·������ͼ���£�
��·������ͼ���£�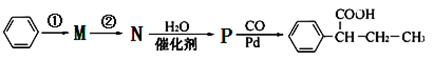
����ٵķ�Ӧ�������Լ�____________������ڵķ�Ӧ����____________��P�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������֬��һ�ֳ�Ĥ�Ժõ���֬��������һ�ִ�����֬�ĺϳ���·��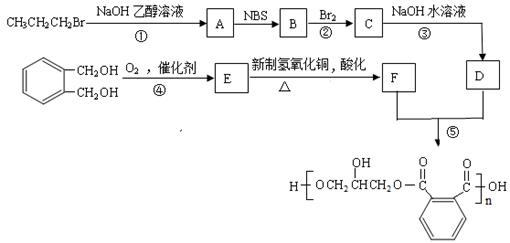
��֪��
��1����Ӧ�ٵĻ�ѧ����ʽ�� ��
��2��д��B�Ľṹ��ʽ�� ��
��3����Ӧ�ܵĻ�ѧ����ʽΪ�� ��
��4����Ӧ�ݵĻ�ѧ����ʽ�� ��
��5������˵����ȷ���� ������ĸ����
a��B����˳���칹
b��1 mol E��������������Һ��Ӧ������2 mol Ag
c��F����NaHCO3��Ӧ����CO2
d�����顢1-������D�зе���ߵ�Ϊ����
��6��д������������������  ��Ϊͬ���칹��Ľṹ��ʽ ��
��Ϊͬ���칹��Ľṹ��ʽ ��
a������Ũ��ˮ��Ӧ���ɰ�ɫ����
b��������һ�ȴ���������
c���ܷ�����ȥ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ճ�ϼ�M�ĺϳ�·������ͼ��ʾ��
���������գ�
��1��д��A��B�Ľṹ��ʽ��A����������B����������
��2��д����Ӧ��������Ӧ��������������Ӧ������������
��3����Ӧ�ۺ͢ݵ�Ŀ����___________________________________________��
��4��C�ľ�����ͬ�����ŵ�ͬ���칹�干�����������֡�
��5��д��D�ڼ���������ˮ��ķ�Ӧ����ʽ��___________________________
__________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������A�Ƿ���ʽΪC7H8O2����Ԫ��״������,���������ֲ�ͬ��ѧ��������,��ԭ�Ӹ�����Ϊ3��1��2��2,���ܷ�������ת��: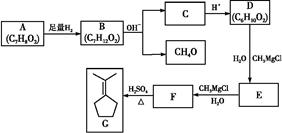
��֪:�ٺ��ʻ��Ļ�����������ʽ�Լ��������·�Ӧ: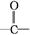
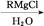
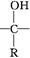
�������ǻ�����ͬһ̼ԭ���ϼ����ȶ�,����ˮ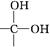
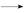
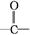 +H2O
+H2O
����������Ϣ,�Իش���������:
(1)д��������A�й����ŵ����� ��
(2)д��������B��F�Ľṹ��ʽB ,F ��
(3)A��B�ķ�Ӧ������ ,F��G�ķ�Ӧ������ ��
(4)��д��D��CH4O��Ӧ�Ļ�ѧ����ʽ ��
��д��F��G�ķ�Ӧ�Ļ�ѧ����ʽ ��
(5)д��������A�ķ�������������ͬ���칹�� ��
�����ڷ����廯���� ������̼������Һ��Ӧ �����ڴ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ϊͬ���칹����л���A��B��C�ķ���ʽ��ΪC5H8O2������AΪֱ���ṹ������ʱ�������Ƶ�������ͭ��Һ��Ӧ������ש��ɫ���ʣ�A�����к˴Ź�������ͼ��������壬�ҷ����֮��Ϊ2��1��1��BΪ��Ԫ������C�ĺ�����ױ���������д��ڼ���A ~ I���л����ת����ϵ���£�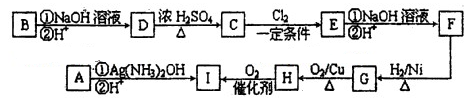
��֪��ϩ����һ�������¿���±�ص��ʷ�������Hԭ�ӵ�ȡ����Ӧ��
��1��A��B�Ľṹ��ʽ�ֱ��ǣ�A_______________��B_______________��
��2��CΪ��ʽ�ṹ����ṹʽΪ_______________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
O��C��_____________________________________________��
G��H��____________________________________________________��
��4��д�����з�Ӧ���ͣ�
F��G_______________��A��I�Ģ�_______________��
��5��д��F�����Ӿ۷�Ӧ���ɵIJ���Ľṹ��ʽ____________________________��
��6��д����A��Ϊͬϵ�������һ�����ʵĽṹ��ʽΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����(CH3)3COH�ṹ���ƵĴ����ܱ������ɶ�Ӧ��ȩ�����ᡣ������������AΪ��ʼ��Ӧ��ϳ�PMAA��·�ߣ�
����д���пհף�
(1)A�Ľṹ��ʽΪ�� ��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
E��F�� .
F��PMAA�� ��
(3)E����Ũ���Ტ���ȵ������£���������F�⣬����������һ�ַ�������һ����Ԫ�����л���G��G�Ľṹ��ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�߷�����άE�㷺����ͨѶ����������������÷�����AΪԭ�����ϳɣ��ϳ�·�����£�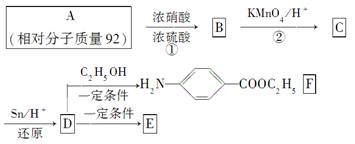
��ش�
(1)д���ṹ��ʽ��A__________��B____________��
C____________��
(2)д����Ӧ���ͣ���__________����_______________________________��
(3)д��D��E�ۺϵĻ�ѧ����ʽ___________________��
(4)���л�����������F������ѧ��Ӧ����________��
a��HCl b��NaCl c��Na2CO3 d��NaOH
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com