
(14��)�����£���һ��Ũ�ȵ�Na2CO3��Һ����CuSO4��Һ�еõ�����ɫ������Ϊ��ȷ���ó�������ɣ�ij�о���ѧϰС�����������ʵ����о���
(һ)�������˵��
����һ�����߷�Ӧֻ����CuCO3������
����������߷�Ӧֻ����Cu(OH)2������
�������� ��
(��)������̽����
�������ֻ����Cu(OH)2��ԭ���� (��һ�����ӷ���ʽ��ʾ)��
�������ϣ�CuCO3��Cu(OH)2�������ᾧˮ��
(��)��ʵ��̽����
����һ����CuSO4��Һ���뵽��Ũ�ȵ������Na2CO3��Һ�в����裬����ɫ�������ɡ�
�����������������Һ�з��������
��������������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
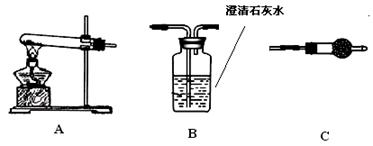
��1����װ������˳��Ϊ ��
��2��װ��C��װ���Լ��������� ��
��3����֤������������CuCO3��ʵ�������� ��

�����ģ� ��������������ͼ��ʾװ�ã����ж���������
��4����Aװ���в��������������� ��
��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ŀ��������÷ֱ���
�� ��
���ݼ�¼��
|
|
Bװ������Ʒ������(g) |
Cװ�õ�����(g) |
Dװ�õ�����(g) |
|
ʵ��ǰ |
33.3 |
262.1 |
223.8 |
|
����� |
24 |
264.8 |
230.4 |
(��)��ʵ����ۡ�
�����������ݳ����жϸó����ijɷ���CuCO3��Cu(OH)2�������ó����Ǵ���������ǻ���ͨ������д�������ʵĻ�ѧʽ ��
��14�֣�
(һ)�������˵�������ɵ���CuCO3��Cu(OH)2�Ļ���1�֣�
(��)������̽������Cu2++CO32-+H2O=Cu(OH)2��+CO2����2�֣���
(��)��ʵ��̽��������1��A��C��B ��2�֣�
��2����ˮ����ͭ ��2�֣�
��3��װ��B�г���ʯ��ˮ����ǣ�2�֣�
��4����U �ιܣ���1�֣�
�ڽ�װ����ԭ�к�ˮ������CO2�Ŀ����ų�����1�֣�
��B�зֽ������ˮ������CO2ȫ���ų�����C��Dװ����ȫ���ա���1�֣�
(��)��ʵ����ۡ���Cu2(OH)2CO3 ��2�֣���
��������
������������ݼ���1�ͼ���2�Ƴ�����3��������ͭӦΪˮ����
���㣺̽�����ʵ���ɻ�������ʵĺ��� ͭ����������Ҫ���������Ҫ����
���������������ѶȽϴ��ۺ��Խ�ǿ�������ƶ��⣬��ɴ�����Ŀ��Ҫע�����������ṩ����Ϣ����ȷ�������ʵ����ʣ��Լ���Щ���ض�ʵ������Ӱ�죬�������ʵ�����ݣ���ϸ���𣬻�Ҫע��ƽʱ֪ʶ�Ļ��ۡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14�֣�.A��B��C�� D�� E������Һ�ֱ���NaOH�� NH3��H2O�� CH3COOH ��HCl ��NH4HSO4�е�һ�֡������½�������ʵ�飺
�ٽ�1 L pH=3��A��Һ�ֱ���0.001mol��L��1 xL B��Һ��0.001mol��L��1 yL D��Һ��
�ַ�Ӧ�����ԣ�x��y��С��ϵΪ�� y��x��
��Ũ�Ⱦ�Ϊ0.1mol��L��1A��E��Һ��pH��A��E��
��Ũ�Ⱦ�Ϊ0.1mol��L��1C��D��Һ�������ϣ���Һ�����ԡ�
�ش��������⣺
��1��D�� ��Һ���ж������� ��
(2) ��ˮϡ��0.1 mol��L��1Bʱ����Һ������ˮ�������Ӷ���С���� (��д���)
��![]() �� ��
�� �� ![]() ��
��
�� c��H+����c��OH-���ij˻� �� OH�������ʵ���
��3��OH��Ũ����ͬ�ĵ������������ҺA��E���ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ��������˵����ȷ����________(��д���)
�ٷ�Ӧ����Ҫ��ʱ��E>A �ڿ�ʼ��Ӧʱ������A>E
�۲μӷ�Ӧ��п�����ʵ���A=E �ܷ�Ӧ���̵�ƽ������ E>A
��A��Һ����п��ʣ�� ��E��Һ����п��ʣ��
��4����������������ʵ���Ũ��B��C��Ϻ���Һ�������¶ȣ����ʲ���ֽ⣩��ҺpH���¶ȱ仯����ͼ�е�_________����(��д���) ��
��5�������£���0.01mol��L��1 C��Һ�еμ�0.01mol��L��1 D��Һ�����ԣ��õ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�ϰ�һ�и�����ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(14��)�����£���һ��Ũ�ȵ�Na2CO3��Һ����CuSO4��Һ�еõ�����ɫ������Ϊ��ȷ���ó�������ɣ�ij�о���ѧϰС�����������ʵ����о���
(һ)�������˵��
����һ�����߷�Ӧֻ����CuCO3������
����������߷�Ӧֻ����Cu(OH)2������
�������� ��
(��)������̽����
�������ֻ����Cu(OH)2��ԭ���� (��һ�����ӷ���ʽ��ʾ)��
�������ϣ�CuCO3��Cu(OH)2�������ᾧˮ��
(��)��ʵ��̽����
����һ����CuSO4��Һ���뵽��Ũ�ȵ������Na2CO3��Һ�в����裬����ɫ�������ɡ�
�����������������Һ�з��������
��������������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�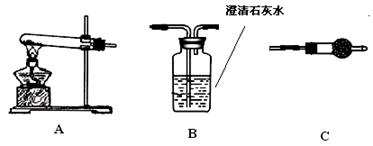
��1����װ������˳��Ϊ ��
��2��װ��C��װ���Լ��������� ��
��3����֤������������CuCO3��ʵ�������� ��
�����ģ� ��������������ͼ��ʾװ�ã����ж���������
��4����Aװ���в��������������� ��
��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ŀ��������÷ֱ���
�� ��
���ݼ�¼��
| | Bװ������Ʒ������(g) | Cװ�õ�����(g) | Dװ�õ�����(g) |
| ʵ��ǰ | 33.3 | 262.1 | 223.8 |
| ����� | 24 | 264.8 | 230.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꼪��ʡ�����и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(14��) ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���
��1��������� �ٸ÷�Ӧ�����������CO2��
�ڸ÷�Ӧ�����������CO��
�۸÷�Ӧ����������� ��
��3����Ʒ��� ��ͼ��ʾ����һ�������������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�
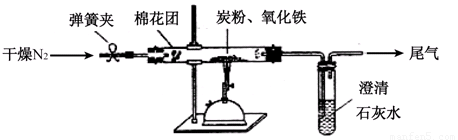
��3����������
��������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO2��
������Һ��ϼ��ȷ�Ӧ�Ƶõ�����
��д���÷�Ӧ�����ӷ���ʽ�� ��
��4��ʵ�鲽��
�ٰ���ͼ����װ�ã������װ�õ������ԣ���ȡ3.20g��������2.00g̼�ۻ�Ͼ��ȣ�����48.48g��Ӳ�ʲ������У�
�ڼ���ǰ����ͨһ��ʱ�䴿������ĵ�����
��ֹͣͨ��N2�н����ɼУ�����һ��ʱ�䣬����ʯ��ˮ������������ǣ�
�ܴ���Ӧ�������ٻ���ͨ��һ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52.24g��
�ݹ��˳�ʯ��ˮ�еij�����ϴ�ӡ���ɺ�Ƶ�����Ϊ2.00g��
����ڡ����ж��ֱ�ͨ��N2�������÷ֱ�Ϊ ��
��5�����ݴ���
�Ը���ʵ�����ݷ�����д����ʵ������������̼������Ӧ�Ļ�ѧ����ʽ��
��
��6��ʵ���Ż� ѧϰС����ͬѧ��ΪӦ��ʵ��װ�ý�һ�����ơ�
�ټ�ͬѧ��Ϊ��Ӧ������ʯ��ˮ����Ba(OH)2��Һ���������� ��
�ڴӻ��������ĽǶȣ����������һ���Ż���������ʵ��װ�ý�һ�����ƣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꼪��ʡ�����и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
(14�֣�.A��B��C�� D�� E������Һ�ֱ���NaOH�� NH3��H2O�� CH3COOH ��HCl ��NH4HSO4�е�һ�֡������½�������ʵ�飺
�ٽ�1 L pH=3��A��Һ�ֱ���0.001mol��L��1 xL B��Һ��0.001mol��L��1 yL D��Һ��
�ַ�Ӧ�����ԣ�x��y��С��ϵΪ�� y��x��
��Ũ�Ⱦ�Ϊ0.1mol��L��1A��E��Һ��pH��A��E��
��Ũ�Ⱦ�Ϊ0.1mol��L��1C��D��Һ�������ϣ���Һ�����ԡ�
�ش��������⣺
��1��D�� ��Һ���ж������� ��
(2) ��ˮϡ��0.1 mol��L��1Bʱ����Һ������ˮ�������Ӷ���С���� (��д���)
�� ��
��
��
�� 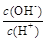 ��
��
�� c��H+����c��OH-���ij˻� �� OH�������ʵ���
��3��OH��Ũ����ͬ�ĵ������������ҺA��E���ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ��������˵����ȷ����________(��д���)
�ٷ�Ӧ����Ҫ��ʱ��E>A �ڿ�ʼ��Ӧʱ������A>E
�۲μӷ�Ӧ��п�����ʵ���A=E �ܷ�Ӧ���̵�ƽ������ E>A
��A��Һ����п��ʣ�� ��E��Һ����п��ʣ��
��4����������������ʵ���Ũ��B��C��Ϻ���Һ�������¶ȣ����ʲ���ֽ⣩��ҺpH���¶ȱ仯����ͼ�е�_________����(��д���) ��
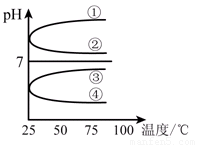
��5�������£���0.01mol��L��1 C��Һ�еμ�0.01mol��L��1 D��Һ�����ԣ��õ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ_______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com