
��ͼ��ʾΪ����ʯ(��ѧʽΪNa3AlF6)�ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�
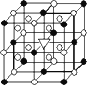
(1)ͼ�С�ֱ�ָ����������________��________��������������Ĵ�������������________������ʯ�ڻ��������е���;________��
(2)H2S��H2O2����Ҫ�������ʱȽ����£�

H2S��H2O2����Է�������������ͬ����������������ʲ������Ҫԭ��________��
(3)��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�������뼫�Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��壮
д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ________����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ����________(��Ԫ�ط���)��ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ��������________��ʵ������м���C2H5OH��ɹ۲쵽��������ɫCu(NH3)4SO4��H2O���壮ʵ��������C2H5OH��������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�
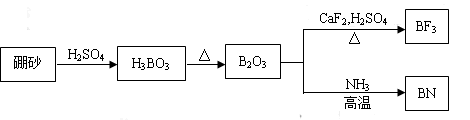
I. ��6�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
��ش��������⣺
(1)��B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ ��
��
(2) BN��BԪ�صĻ��ϼ�Ϊ ��
II. ��4�֣���ҵ����ȡ����ʯ(Na3AlF6)�Ļ�ѧ����ʽ���£�
2Al(OH)3+12HF+3Na2CO3=2Na3AlF6+3CO2��+9H2O
�����������գ�
(3����Ӧ����������Ԫ����Ԫ�����ڱ���λ�����ڣ��������ж����ǵĽ����Ի�ǽ�����ǿ������ (ѡ����ĸ����
A����̬�⻯����ȶ��� B������������Ӧˮ���������
C��������������Ӧ������ D��������ͬŨ���ᷢ����Ӧ�Ŀ���
(4��2004�꣬������ѧ��ͨ������������̫��̽������Ǵ����к���һ�ֳ�Ϊ���ʣ���ѧʽΪCOS�������ʡ���֪�����������̼�Ľṹ���ƣ�����ԭ�ӵ�����㶼����8���ӽṹ�����������л�ȼ�ա���д�����ʵĵ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ������ʮһ���и�һ��ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ������
��10�֣�
I.��6�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
��ش��������⣺
(1)��B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ ��
��
(2) BN��BԪ�صĻ��ϼ�Ϊ ��
II. ��4�֣���ҵ����ȡ����ʯ(Na3AlF6)�Ļ�ѧ����ʽ���£�
2Al(OH)3+12HF+3Na2CO3=2Na3AlF6+3CO2��+9H2O
�����������գ�
(3����Ӧ����������Ԫ����Ԫ�����ڱ���λ�����ڣ��������ж����ǵĽ����Ի�ǽ�����ǿ������ (ѡ����ĸ����
| A����̬�⻯����ȶ��� | B������������Ӧˮ��������� |
| C��������������Ӧ������ | D��������ͬŨ���ᷢ����Ӧ�Ŀ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�켪��ʡ�����и�һ��ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
��10�֣�
I. ��6�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
��ش��������⣺
(1)��B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ ��
��
(2) BN��BԪ�صĻ��ϼ�Ϊ ��
II. ��4�֣���ҵ����ȡ����ʯ(Na3AlF6)�Ļ�ѧ����ʽ���£�
2Al(OH)3+12HF+3Na2CO3=2Na3AlF6+3CO2��+9H2O
�����������գ�
(3����Ӧ����������Ԫ����Ԫ�����ڱ���λ�����ڣ��������ж����ǵĽ����Ի�ǽ�����ǿ������ (ѡ����ĸ����
A����̬�⻯����ȶ��� B������������Ӧˮ���������
C��������������Ӧ������ D��������ͬŨ���ᷢ����Ӧ�Ŀ���
(4��2004�꣬������ѧ��ͨ������������̫��̽������Ǵ����к���һ�ֳ�Ϊ���ʣ���ѧʽΪCOS�������ʡ���֪�����������̼�Ľṹ���ƣ�����ԭ�ӵ�����㶼����8���ӽṹ�����������л�ȼ�ա���д�����ʵĵ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ̩���и�����һ��ģ�⿼�Ի�ѧ�� ���ͣ�ʵ����
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ�����С��⣬������Ӧ�Ĵ����������������ⶼ������A�����֡�
A��þ��ͭ�Ƚ��������������ڶ���ø�ĸ����ӡ���ҵ�ϴӺ�ˮ����ȡþʱ�����Ʊ���ˮ�Ȼ�þ��Ȼ�������ڵ�⣬�õ�����þ��
��1����ο�����������պͻش����⣺

��ҵ�ϳ��õ������MgCl2�ķ�����������þ�����Al2O3�����ʯ���ڻ����ķ��������������õ��MgO�ķ�������þ��ԭ�� �����õ��AlCl3�ķ�����������ԭ�� ��
��2��2001������������þ������ˢ���˽��������ﳬ���¶ȵ����¼���û��Ͼ���ṹ�еľ�����ͼ��ʾ��þԭ�Ӽ��γ�����������������ԭ��λ�������ڡ���û�����Ļ�ѧʽ�ɱ�ʾΪ ��

��3��д��Cu+�ĺ�������Ų�ʽ ��
��4��������ͭ��Һ�м��������ˮ��������[Cu��NH3��4]2+�����ӡ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ���� ��
��5��ij���ͪ��ҩ�����������Cu2�����ṹ����ͼ�����ڸ�ҩ���˵����ȷ���� ��

A����������Cu2+�������5
B��Nԭ�Ӿ�����sp2�ӻ�
C��������λ�������Թ��ۼ��ͷǼ��Թ��ۼ�
D���۵�ܸߣ�Ӳ�Ⱥܴ�
B��ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
����I����ȡ0��4 g��������Ʒ��������������ĵ�ƿ����ͼ���ڣ�����10 mL���Ȼ�̼������ҡ��ʹ��ȫ���ܽ⡣���ƿ�м���25��00 mL��0��01 mol IBr����ˮ������Һ���Ǻ�ƿ�����ڲ�������ƿ��֮��μ�����10���⻯����Һ��շ�϶������IBr�Ļӷ���ʧ��

����II���ڰ�������30 min������ʱ����ҡ����30 min��С�ĵش��������������Ƶ�10��
�⻯��10 mL������ˮ50 mL�Ѳ�������ƿ���ϵ�Һ���ϴ��ƿ�ڡ�
�������ָʾ������0��1 mol��L��1�����������Һ�ζ���������ƿ��ֱ���յ㡣
�ⶨ�����з�������ط�Ӧ���£�
��
��IBr��KI=I2+KBr
��I2��2S2O32��=2I����S4O62��
��ش��������⣺
��1����֪±�ػ�����IBr��������±�ص������ƣ�ʵ����ȷ��ȡIBr��ҺӦѡ�õ������� ����ƿ������ᷢ����Ӧ�Ļ�ѧ����ʽ ��
��2��������е�ƿ�ڰ�������30 min������ʱ����ҡ����ԭ���� ��
��3�������������ָʾ��Ϊ ���ζ��յ������ ��
��4����Ӧ�������Һ�������л������Ȼ�̼����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ�����С��⣬������Ӧ�Ĵ����������������ⶼ������A�����֡�
A��þ��ͭ�Ƚ��������������ڶ���ø�ĸ����ӡ���ҵ�ϴӺ�ˮ����ȡþʱ�����Ʊ���ˮ�Ȼ�þ��Ȼ�������ڵ�⣬�õ�����þ��
��1����ο�����������պͻش����⣺
��ҵ�ϳ��õ������MgCl2�ķ�����������þ�����Al2O3�����ʯ���ڻ����ķ��������������õ��MgO�ķ�������þ��ԭ�� �����õ��AlCl3�ķ�����������ԭ�� ��
��2��2001������������þ������ˢ���˽��������ﳬ���¶ȵ����¼���û��Ͼ���ṹ�еľ�����ͼ��ʾ��þԭ�Ӽ��γ�����������������ԭ��λ�������ڡ���û�����Ļ�ѧʽ�ɱ�ʾΪ ��
��3��д��Cu+�ĺ�������Ų�ʽ ��
��4��������ͭ��Һ�м��������ˮ��������[Cu��NH3��4]2+�����ӡ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ���� ��
��5��ij���ͪ��ҩ�����������Cu2�����ṹ����ͼ�����ڸ�ҩ���˵����ȷ���� ��
A����������Cu2+�������5
B��Nԭ�Ӿ�����sp2�ӻ�
C��������λ�������Թ��ۼ��ͷǼ��Թ��ۼ�
D���۵�ܸߣ�Ӳ�Ⱥܴ�
B��ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
����I����ȡ0��4 g��������Ʒ��������������ĵ�ƿ����ͼ���ڣ�����10 mL���Ȼ�̼������ҡ��ʹ��ȫ���ܽ⡣���ƿ�м���25��00 mL��0��01 mol IBr����ˮ������Һ���Ǻ�ƿ�����ڲ�������ƿ��֮��μ�����10���⻯����Һ��շ�϶������IBr�Ļӷ���ʧ��
����II���ڰ�������30 min������ʱ����ҡ����30 min��С�ĵش��������������Ƶ�10��
�⻯��10 mL������ˮ50 mL�Ѳ�������ƿ���ϵ�Һ���ϴ��ƿ�ڡ�
�������ָʾ������0��1 mol��L��1�����������Һ�ζ���������ƿ��ֱ���յ㡣
�ⶨ�����з�������ط�Ӧ���£�
��
��IBr��KI=I2+KBr
��I2��2S2O32��=2I����S4O62��
��ش��������⣺
��1����֪±�ػ�����IBr��������±�ص������ƣ�ʵ����ȷ��ȡIBr��ҺӦѡ�õ������� ����ƿ������ᷢ����Ӧ�Ļ�ѧ����ʽ ��
��2��������е�ƿ�ڰ�������30 min������ʱ����ҡ����ԭ���� ��
��3�������������ָʾ��Ϊ ���ζ��յ������ ��
��4����Ӧ�������Һ�������л������Ȼ�̼����������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com