
 ��
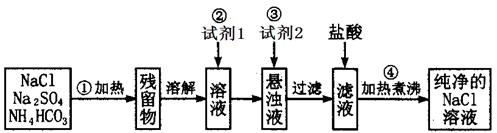
�� �ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣
�ڽ���Ʒ��ˮ�ܽ⣬�Ƴɴ�����Һ�����������Һ�м��������ij���Լ������ˣ��ܳ���ϴ�Ӻ�С�ĺ�ɣ��õ���������A������Һ�ڽ���ijһ�����������������������õ���������B������ʵ���еõ���ij�ֹ��塣 ��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� ��
��������ͬ����Ŀ�IJ�ͬ���ڲ�����е�Ŀ�� ���ڲ�����е�Ŀ���� �� ���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��
���ǹ���B�� ����A��B�����㲻ѡ����һ�ֹ���������� ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥCO2�е�HCl���壬ͨ��ʢ��������NaOH��Һ��ϴ��ƿ |
| B������KNO3�����е�����NaCl���ܽ�������ᾧ������ |
| C���ü��ȷ������Ȼ��ƺ͵�Ĺ������� |
| D��������������Һ�е������ƣ����������Ȼ�����Һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
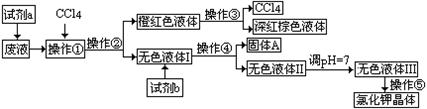
 H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��| A����ȡ�����ˡ���Һ�����ˡ������ᾧ | B����ȡ����Һ�������ˡ������ᾧ |
| C����Һ����ȡ�����ˡ����ˡ������ᾧ | D����ȡ����Һ����Һ�����ˡ������ᾧ |
 �������� ������������ ��
�������� ������������ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥN2�е�����O2��ͨ�����ȵ�CuO��ĩ���ռ����� |
| B����ȥCO2�е�����HCl��ͨ��Na2CO3��Һ���ռ����� |
| C����ȥNaCl��Һ������CaCl2����������Na2CO3������ |
| D����ȥKCl��Һ������MgCl2����������NaOH��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һ����ȡ������ | B����ȡ������Һ |
| C����Һ��������ȡ | D��������ȡ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B������ʱ��Ӧʹ�¶ȼƵ�ˮ����������ƿ��ƿ�� |
| C������ʱ���������¶�������������Ӧ����������ֽ�� |
| D������ʱ������������������ƽ���̣����������ƽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ������ȷ����
������ȷ����| A���ö����ЧӦ����Fe(OH)3�����FeCl3��Һ |
| B���ù��˵ķ�����ȥNaCl��Һ�к��е��������۽��� |
| C�����ܽ⡢���˵ķ����ᴿ��������BaSO4��BaCO3 |
| D���ü��ȡ������ķ������Գ�ȥ�����е�CaCl2��MgCl2������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com