
Ϊȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�顣
��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊb L����Ӧ�Ļ�ѧ����ʽ��__________________________����Ʒ������������_____________g��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�_____________����������������������_____________��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L���������루1������������������c��b��_____________��
��1��2Al��2NaOH��6H2O��2Na[Al(OH)4]��3H2����9b/11.2
��2�� 2Al��Fe2O3![]() Al2O3��2Fe�� 80��27 ��3�� 2��3
Al2O3��2Fe�� 80��27 ��3�� 2��3
��1����Ʒ�е�����������NaOH��Һ��Ӧ����������NaOH��Һ��Ӧ�������ǻ��������ƺ�������
2Al �� 2NaOH �� 6H2O �� 2Na[Al(OH)4] �� 3H2��
2��27g��54g 3��22.4L��67.2L
m(Al) b L
m(Al)��b L��67.2L��54g��9b/11.2 g
��2�������зdz�ǿ�Ļ�ԭ�ԣ��ڵ�ȼ�����¿��������������ҷ�Ӧ�����������������ʣ�2Al��Fe2O3![]() Al2O3��2Fe���������ϻ�ѧ����ʽ��������������������������Ϊ80��27��
Al2O3��2Fe���������ϻ�ѧ����ʽ��������������������������Ϊ80��27��
��3�������ȷ�Ӧ�ķ���ʽ�����������������ʵ�������ȵģ���������ķ�Ӧ�У����ļ�̬���ߵ�+3�ۣ����ļ�ֻ̬���ߵ�+2�ۡ���ˣ�c��b��2��3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 18b |
| 22.4 |
| 18b |
| 22.4 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊb L����Ӧ�Ļ�ѧ����ʽ��____________________����Ʒ������������________g��
��2����ȡa g��Ʒ�����ȼ��ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�________________________________����������������������________��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L���������루1������������������c��b=________��?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�Ϊȷ��ij���ȼ���������������������ɣ��ֱ��������ʵ�顣
��1����ȡ![]() g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊ
g��Ʒ�������м���������NaOH��Һ��������ɵ����壨��״������ͬ�����Ϊ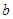 L����Ӧ�Ļ�ѧ����ʽ��_________________����Ʒ������������____________g��
L����Ӧ�Ļ�ѧ����ʽ��_________________����Ʒ������������____________g��
2����ȡ![]() g��Ʒ�ڸ����½���ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�__________________����������������������____________��
g��Ʒ�ڸ����½���ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�__________________����������������������____________��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ������Ϊ![]() L���������루1������������������
L���������루1������������������![]() ��_________��
��_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com