
| |||||||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 п�̸ɵ��������ʹ�õĻ�ѧ��أ������������ͼ��ʾ��
п�̸ɵ��������ʹ�õĻ�ѧ��أ������������ͼ��ʾ��| ʵ�鲽�� | ʵ������ | ʵ����ۺͽ��� | ||||||||
| ȡ����������ɫ��Һ���� ���У���μ���NaOH��Һ��ֱ���������ټ��� | ���ɰ�ɫ������ ��ɫ������ʧ�� ��ɫ������ʧ�� �����д̼�����ζ������ �����д̼�����ζ������ |
��ɫ��Һ�д���Zn2+��NH4+���� ����NH4+���Ӵ��ڵ����ӷ���ʽ�� NH4++OH-
NH4++OH-
|

| ��� | ��ƿ�е����� | ʵ���¼ | ʵ���������� |
| ʵ��һ | ����MnO2 | �ռ���56mL���� | MnO2������ |
| ʵ��� | ����MnO2��ϡ���� | ��ɫ��ĩ�����ܽ⣬ �ռ���112mL���� |
MnO2��������������Mn2+���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
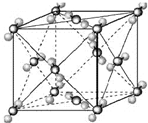 ��2009?��Ǩģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ������ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��2009?��Ǩģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ������ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��1���ϳɰ���Ӧ�������������������� ����2 L�ܱ�������ͨ��2 mol��N2��6 mol��H2��5 minʱ��Ӧ�ﵽƽ�⣬��ʱ�����ڵ�ѹǿ��Ϊԭ����3/4��0��5min�÷�Ӧ�Ļ�ѧ��Ӧ����ΪV(H2)=���������� ��N2��ת����=���������� ����ѧƽ�ⳣ��K=���������� ��
��2��NH3 (g)��CO2(g)����������Ӧ�������ء�CO(NH2)2����������Ӧ�������仯ʾ��ͼ���£�

��NH3(g) ��CO2(g) ��Ӧ�������ص��Ȼ�ѧ����ʽΪ�������������������������� ��
��3����CO�ϳɼ״���CH3OH���Ļ�ѧ����ʽΪ![]() ��
��
 ������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ������ͼ��ʾ������˵����ȷ���������� ������ĸ��
������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ������ͼ��ʾ������˵����ȷ���������� ������ĸ��
�� A���¶ȣ�T1 > T2 > T3������������
![]() B��ƽ�ⳣ����
B��ƽ�ⳣ����
![]() C��ƽ�ⳣ������������������������
C��ƽ�ⳣ������������������������
![]() �� D������Ӧ���ʣ�
�� D������Ӧ���ʣ�
![]() E. ƽ��Ħ��������
E. ƽ��Ħ��������
(4)��25���£���a mol��L��1�İ�ˮ��0.01 mol��L��1������������ϣ���Ӧƽ��ʱ��Һ��C(NH+4)=C(Cl?)������Һ�ԣ����� ���ԣ���ᡱ������С������ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb=������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ��Ǩ�и߿���ѧģ���Ծ���һ���������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com