
| A���٢� | B���ۢ� | C���ڢ� | D���ݢ� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ��ɳ�и����ڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и���������ָ����Һ�У����ܴ���������ǣ� ��
������������������ɫ��Һ�У�K+��NH ��Na+��HSO
��Na+��HSO ��PO
��PO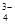 ��MnO
��MnO
����ʹpH��ֽ������ɫ����Һ�У�K+��Na+��AlO ��NO
��NO ��S
��S ��SO
��SO
��ˮ�����H+Ũ��c(H+)=10-13mol��L-1����Һ�У�Cl ��CO
��CO ��S2O
��S2O ��Fe3+��K+��Na+
��Fe3+��K+��Na+
�ܼ���Al�ܷų�H2����Һ�У�Na+��NH ��K+��SO
��K+��SO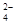 ��Cl
��Cl ��Br
��Br
��ʹʯ�������Һ�У�Fe3+��Na+��K+��NO ��SO
��SO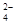 ��Cl
��Cl
����������pH=1����Һ�У�Fe2+��Al3+��K+��Na+��NO ��I
��I ��Cl
��Cl ��S
��S
�ߺ��д���Fe3+����Һ��K+��NH ��Na+��SCN
��Na+��SCN ��I
��I ��Br
��Br
�������£� =0.1 mol/L����Һ�У�Na+��K+��SO
=0.1 mol/L����Һ�У�Na+��K+��SO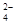 ��SiO
��SiO ��NO
��NO
A���٢ۢܢ� B���٢ۢޢ� C���ڢܢݢ� D���ڢݢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ָ�������У����и�������һ�����Դ�������� ( )
A.ʹpH��ֽ�ʺ�ɫ����Һ: ![]()
B.�����£���![]() ����Ҵ:
����Ҵ:![]()
C.�������۷ų���������Һ��![]()
D.ʹ��ɫ��̪��Һ�Ժ�ɫ����Һ��![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com