
ʵ���⣺
������NaHCO3��������500mL 0.2mol/L��NaHCO3��Һ������������գ� ��1�����Ƹ���ҺӦѡ�� mL����ƿ
��1�����Ƹ���ҺӦѡ�� mL����ƿ ��2����������ƽ��ȡ g����NaHCO3
��2����������ƽ��ȡ g����NaHCO3 ��3�����ƺõ�NaHCO3 �������500mL�Ĵ��ձ��У�����Լ250mL����ˮ���ò�������������ȫ�ܽ⡣����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ��
��3�����ƺõ�NaHCO3 �������500mL�Ĵ��ձ��У�����Լ250mL����ˮ���ò�������������ȫ�ܽ⡣����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ�� ��4������������ˮϴ���ձ�2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע������ƿ������ζ�����ƿ��ʹ��Һ��;��ȡ�
��4������������ˮϴ���ձ�2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע������ƿ������ζ�����ƿ��ʹ��Һ��;��ȡ� ��5��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ2-3����ʱ������ �μ�����ˮ��Һ����̶������С��Ǻ�ƿ����ҡ�ȡ�
��5��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ2-3����ʱ������ �μ�����ˮ��Һ����̶������С��Ǻ�ƿ����ҡ�ȡ� ��6��
��6�� �������ػ����ʵ����ƫ�͵��ǣ� ��
�������ػ����ʵ����ƫ�͵��ǣ� ��
| A������ʱ�۲�Һ������ | B������ʱ�۲�Һ�温�� |
| C��û�еڣ�4������������ˮϴ���ձ� | D������ƿ��ԭ������������ˮ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
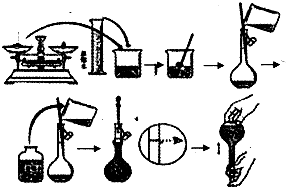 ������NaOH��������100mL 0.5mol/L��NaOH��Һ�����ƹ�����ͼ��ʾ��
������NaOH��������100mL 0.5mol/L��NaOH��Һ�����ƹ�����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
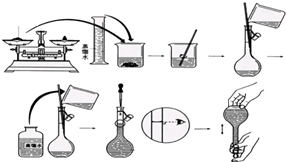 ������NaOH��������100mL 0.5mol/L��NaOH��Һ�����ƹ�����ͼ��ʾ�����������ش�
������NaOH��������100mL 0.5mol/L��NaOH��Һ�����ƹ�����ͼ��ʾ�����������ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������NaOH��������250mL 0.2mol/L��NaOH��Һ��
������NaOH��������250mL 0.2mol/L��NaOH��Һ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com