
��ͼ���ֽ�����������������������ͼ��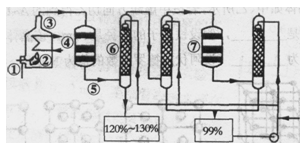
��1���ڢܴ����������������������ܵ��豸������ ���ô�������Ӧ�ķ���ʽΪ ��Ϊ�����������IJ��ʣ��ô�Ӧ���� ������¹��̡����ȹ��̡���Ϊ�ˣ�
��2���ڢߴ����ж��δ�������ԭ���� ��
��3���ݴ�����������Ҫ�ǵ�������������ʱ���徭�����������������Ϊ�� ��
��4��20���ķ������ᣨSO3����������Ϊ20����1�����ˮ �֣�����2λ��Ч���֣��������Ƴ�98���ij�Ʒ���ᣮ
��5���ڢڴ�����1500��ġ�����ȫȼ�ա������Ȼ����������������Ȼ���ڢ۴���700�����ټ���ȼ�գ��Լ�������ȼ�շ�ʽ�Ի���������������ԭ�� ��
��1���Ӵ��� ��2�֣� 2SO2��O2 2SO3��2�֣���V2O5��д�ɡ�������Ҳ�ɣ���������1�֣��ޡ�
2SO3��2�֣���V2O5��д�ɡ�������Ҳ�ɣ���������1�֣��ޡ� �����÷֣������¹��̣�2�֣�
�����÷֣������¹��̣�2�֣�
��2���÷�ӦΪ���淴Ӧ�����δ�ʹ��δ��Ӧ��SO2������������SO3�����Խ��ͳɱ����ԭ�������ʺͱ���������2�֡����С����ԭ�������ʡ�1�֣������������� 1�֣�
��3��ͨ�����������������SO3�����϶࣬������SO2�Ĵ�������Ӧ���С���2�֡���SO3�����϶ࡱ1�֣���������SO2�Ĵ�������1�֣�
��4��0��066��3�֣���0��001���ɵ÷֣�
��5���ڸ����£������ĵ�����������Ӧ���ɵ����������Ⱦ�������£��������ﱻ��ԭ������N2���Ի���������������2�֣������ɵ������1�֣����������ﱻ��ԭ��1�֣�
���������������1��������������������������豸Ϊ�Ӵ��ң�SO2��O2��Ӧ����SO3����ѧ����ʽΪ��2SO2��O2 2SO3���÷�Ӧ�Ƿ��ȵĿ��淴Ӧ���¶ȹ���ƽ�������ƶ��������� SO3 �����ɣ���Ӱ������Ļ��ԣ����Բ��õ��¹��̡�
2SO3���÷�Ӧ�Ƿ��ȵĿ��淴Ӧ���¶ȹ���ƽ�������ƶ��������� SO3 �����ɣ���Ӱ������Ļ��ԣ����Բ��õ��¹��̡�
��2��ͨ��һ�δ�¯�ܲ����ܽ�����������ȫ��������������Ϊ�������ԭ�ϣ��������ö��δ�¯�ߣ�ʹ��δ��Ӧ�Ķ�����������������������������ڽ��ͳɱ��ͻ����������DZ�Ҫ�ġ�
��3���ݴ��Ļ��������Ҫ����������͵����������Ķ�������ͨ����������ֻ������һ�������������������ֺ���������ࡢ���������ٵĻ������ֱ��ͨ��ڶ�����¯�ߣ���ʱ������������ϵ����Ȼ���ķ�ѹ�Ƚϸߣ���������ķ�ѹ�ܵͣ������ڷ�Ӧ���С�
��4��SO3��H2O��Ӧ����H2SO4����ҪH2O������Ϊ��1t��20%��18/80=0.045t��Ũ���Ậˮ������Ϊ����0.8t+0.2t��98/80����2%=0.0209t������һ����Ҫˮ������Ϊ��0.045t+0.0209t ="0.066t" ��
��5��1500��ʱ�������еĵ� ����������Ӧ�����ж��ĵ��������Ⱦ�����¶Ƚϵͣ��� 700���£��������ﱻ��ԭΪN2����������ȼ�շ����Ի�������������
���㣺���⿼�黯ѧ����ͼ�ķ�������ѧ����ʽ����д����ҵ����ԭ��ķ�������Ӧ��ļ��㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���п�ͼ�漰����������Ԫ����,��һ��Ԫ����,�����Ϊ1��18��Ԫ�ء�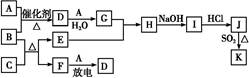
��֪:A��FΪ��ɫ���嵥��,BΪ���д̼�����ζ������,CΪ��ɫ������,EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش���������:
��1��D�Ļ�ѧʽΪ����������������;F�ĽṹʽΪ�� ������
��2��A��B��Ӧ�Ļ�ѧ����ʽΪ�� ��
��3��E��G��ϡ��Һ��Ӧ�����ӷ���ʽΪ�� ��
��4��B��C��Ӧ�Ļ�ѧ����ʽΪ�� ��
��5��J��K��ͬ�ֽ����IJ�ͬ�Ȼ���,KΪ��ɫ������д��SO2��ԭJ����K�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
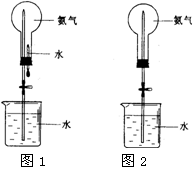
��Ȫ��һ�ֳ�������Ȼ�����������ԭ���Ǵ���ѹǿ���ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� _________ ��
��2���ռ�����Ӧʹ�� ����
��3��Ҫ�õ�����İ�����ѡ������ ���������
| A��Ũ���� | B����ʯ�� | C��NaOH���� | D��P2O5���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣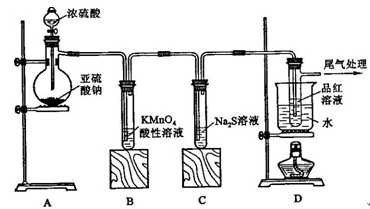
�뵽��F�����⣺
��1��װ��A��ʢ���������Ƶ����������� �����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������У�װ��B��C�з���������ֱ��� �� ����Щ����ֱ�˵��SO2���е������� �� ��װ��B�з�����Ӧ�����ӷ���ʽΪ ��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ����������� ��
��4��β���ɲ��� ��Һ���ա�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�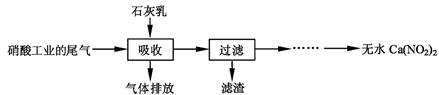
��ش��������⣺
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK= ��
N2O3(g)����ƽ�ⳣ������ʽΪK= ��
��2�����������в�����Һ�����Ӵ�����(β�����������ײ����룬ʯ�������������������)����Ŀ���� ��������ѭ�����ã���������Ҫ�ɷ��� (�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1�U1����n(NO)��n(NO2)��1�U1,��ᵼ�� ����n(NO)��n(NO2)��1�U1,��ᵼ�� ��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ڿƼ���������Ӧ�ù㷺��
��1����ҵ�ϳ�����ʯ�Һ�������Ӧ��ȡƯ�ۣ���ѧ����ʽ�� ��
��2��ʵ������MnO2��Ũ���ᷴӦ��ȡ������ԭ�����£�MnO2 + 4HCl MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O
������ȡ11.2 L Cl2����״������������Ӧ����MnO2������Ϊ______g��
����ƽ���ƶ�ԭ�����Ϳ����ű���ʳ��ˮ���ռ�������ԭ�� ������ϱ�Ҫ�Ļ�ѧ���P���ֻش�
���Ʊ�����ʱ������NaOH��Һ����β���������Լ�Ҳ������������������____������ĸ����
a. KI��Һ b. FeCl2��Һ c. KCl��Һ
д����ѡ��������Լ���Cl2��Ӧ�����ӷ���ʽ��_______��
��Ҳ����Ũ��ˮ����������ͬʱ����һ������Ⱦ�����壬��Ӧ�Ļ�ѧ����ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������;�㷺�Ļ���ԭ�ϣ�������ˮ������ˮ�����������ʹ����ȡ�
��ҵ������ͭ�ķ����ܶࡣ
��1������һ����Ũ�����ͭ��ȡ����ͭ���÷�Ӧ�Ļ�ѧ����ʽ��_____________________,�˷������ȱ����____________________________��
��2������������ϡ���ᡢͭ����������ȡ����ͭ����������Ҫ��������ͼ��ʾ��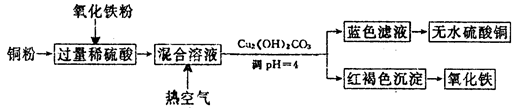
��ϡ���ᡢͭ����������Ӧ�����ӷ���ʽ��__________________��________________��
������Һ��ͨ���ȿ����ķ�Ӧ�����ӷ���ʽ��_______________________________��
����˵������PHΪ4��Ŀ����_______________________������Һ�õ���ˮ����ͭ��ʵ�������______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϴ�������ǹ辧Ƭ��������Ҫ����֮һ����Ƭ��ѧ��ϴ����ҪĿ���dz�ȥ��Ƭ�������ʣ���ijЩ�л�����Σ�������Si��SiO2�۳��ȣ������õĻ�ѧ��ϴ���иߴ�ˮ���л��ܼ���˫��ˮ��Ũ�ᡢǿ��ȡ�����ȥ����������ͨ����һ��Ũ�ȵ�HF��Һ�����������½���Ƭ����1�������ӡ��������ڹ�Ƭ�����γɽ������ε����棬���ӹ��̫��������ա���������ͨ����NaOH��Na2SiO3�Ȼ����Һ��75��90�淴Ӧ25��35 min��Ч�����á�
�ش���������
��1���ܷ��ò����Լ�ƿ��ʢHF��Һ��Ϊʲô?�û�ѧ����ʽ���Խ��� ��
��2��д����Ƭ����Ӧ�����ӷ���ʽ ���Ե�������1990�껯ѧ��Seidel�����һ�ֵĵ绯ѧģ�ͣ���ָ��Si��NaOH��Һ�ķ�Ӧ��������Si��OHһ��Ӧ������SiO44һ��Ȼ��SiO44һѸ��ˮ������H4SiO4�����ڴ�ԭ��������Ӧ��������Ϊ ��
��3����У��ѧ��ȤС��ͬѧ��Ϊ��֤Seidel�������Ƿ���ȷ���������ʵ�飺
| | ʵ����ʵ |
| ��ʵһ | ˮ������600��ʱ��ʹ��ĩ״�軺���������ų������� |
| ��ʵ�� | ʢ���ڲ���ʯӢ�����еĴ�ˮ��ʱ��Է�ĩ״��ԭ����ʴ���á� |
| ��ʵ�� | ��ͨ���������е�ˮ�����дӲ������ܳ������ļ���ʹ��ĩ״�������л����ܽ⡣ |
| ��ʵ�� | ��Ұ�����ýϸ߰ٷֱȵĹ�����������Ca(OH)2��NaOH�����ź����գ��ɾ��ҷų�H2�� |
| ��ʵ�� | 1g��0.036mo1��Si��20mL����lgNaOH��0.025mol������Һ��С�ļ��ȣ���Ԥ�ȣ����ռ���Լ1700mL H2���ܽӽ�����ֵ��1600mL���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij����M�����������ˮ�Ͼ���[M(OH)2?xH2O]��Na2CO3����ﹲ36.800g������������ˮ������MCO3�İ�ɫ�������������˳���ϴ����ɣ�������Ϊ9.850g��
47. �� 9.850g MCO3�������������أ��õ�7.650g MO���壬�����CO2����_______mol��
48����Һ�������ò��������壻�����������������Һ���ȣ������4.48L����(��״��)����Һ��OH?�����ʵ���Ϊ_______mol��
49.M�����ԭ������Ϊ_________����ͨ������ȷ��M(OH)2?xH2O��x��ֵ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com