
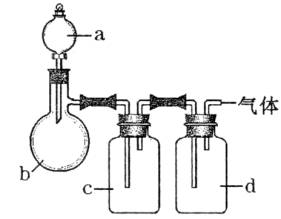
| ���� | a | b | c | d |
| C2H4 | �Ҵ� | ŨH2SO4 | NaOH��Һ | ŨH2SO4 |
| Cl2 | Ũ���� | MnO2 | NaOH��Һ | ŨH2SO4 |
| NH3 | ����NH4Cl��Һ | ��ʯ�� | H2O | ����NaOH |
| NO | ϡHNO3 | ͭм | H2O | P2O5 |


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��� | ��ѡ�������� | �������� |
| �ڢ��� | | |
| �ڢ��� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �������������ʵ�����Ʒ����������������ա�
�������������ʵ�����Ʒ����������������ա�
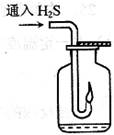
 �������շ�������ȡ�������壬����ȡ�����ԭ�Ͽ�ѡ��________��
�������շ�������ȡ�������壬����ȡ�����ԭ�Ͽ�ѡ��________�� a��ϡ������������ b��ϡ������������
a��ϡ������������ b��ϡ������������ c��ϡ���������� d��ϡ������������
c��ϡ���������� d��ϡ������������ С��2:��Ҫ��װһ���Կ�����������������ʵ�װ�ã�������ͼ��ѡ����ʵ�������
С��2:��Ҫ��װһ���Կ�����������������ʵ�װ�ã�������ͼ��ѡ����ʵ������� ______________�����ţ���
______________�����ţ���




 С��3:����ͼ����һ�����������ļ���ƿ�м�������Ʒ��ϡ����Һ��
С��3:����ͼ����һ�����������ļ���ƿ�м�������Ʒ��ϡ����Һ�� ��ȼ�������塣�ڻ����Զ�Ϩ���ֹͣͨ�����壬ƿ�ڿɹ۲쵽
��ȼ�������塣�ڻ����Զ�Ϩ���ֹͣͨ�����壬ƿ�ڿɹ۲쵽 ��������___________________________________________________��
��������___________________________________________________�� С��4:����������ƿ�м���ͨ���������壬��������Ӧ�Ļ�ѧ����ʽΪ��
С��4:����������ƿ�м���ͨ���������壬��������Ӧ�Ļ�ѧ����ʽΪ�� ______________________________________________________________
______________________________________________________________ ��Ӧ�����У���Һ��pH______����������С�����䡱����
��Ӧ�����У���Һ��pH______����������С�����䡱���� С��5:��ȼ����������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
С��5:��ȼ����������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��

 С��6:��֪���������ڿ����е��������Ϊ4.3%��45.5%ʱ�ᷢ����ը�������������ڿ����е��������Ϊ30%ʱ���䱬ը������______________________��
С��6:��֪���������ڿ����е��������Ϊ4.3%��45.5%ʱ�ᷢ����ը�������������ڿ����е��������Ϊ30%ʱ���䱬ը������______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��88 | B��102 | C��116 | D��196 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com