
��ͼ��ʾΪһ���л��ϳɷ�Ӧ������ͼ�� 
�����ͼʾ�ش��������⣺
д������1��д���м����A��B��D�Ľṹ��ʽ��
A�� B�� D��
��2����ͼ�Т����ߵķ�Ӧ������ȡ����Ӧ���� ���� ����ţ�
��3��д��C��ͬ���칹��X��Ҫ�������������������
����������������������
����1molX������NaHCO3��Һ��Ӧ����1molCO2���塣
д������������������X�����нṹ��ʽ ��
д������һ��X��NaOH��Һ����ʱ�ķ�Ӧ����ʽ ��
��1��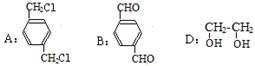
��2���٢ڢޢ�
��3��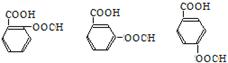
��4��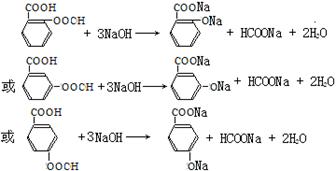
���������������1�� �ڹ��յ�����������������ȡ����Ӧ����A��
�ڹ��յ�����������������ȡ����Ӧ����A��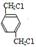 ��
��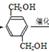 ��������������B��
��������������B��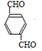 ��B�ڴ���������������C
��B�ڴ���������������C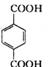 ����
����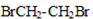 �ڼ���Һ��������ˮ������D��
�ڼ���Һ��������ˮ������D��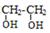 ����2���٢ڢޢ߾�Ϊȡ����Ӧ����3��������������������
����2���٢ڢޢ߾�Ϊȡ����Ӧ����3��������������������
1molX������NaHCO3��Һ��Ӧ����1molCO2����˵������������������һ�����Ȼ�һ����֬�����ʷ���������������X�����нṹ��ʽΪ��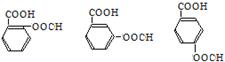 ����4����ͬ���칹�����������Ƶķ�Ӧ���£�
����4����ͬ���칹�����������Ƶķ�Ӧ���£�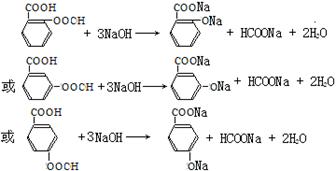
���㣺�л��ƶ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
PMAA��һ�֡������͡�����ӣ�������������ҩ�д������С���ӵķ��룬���л�����(�ۼ���ϩ�����)�����ɻ��������ȵĴ�����������������������������AΪ��ʼ��Ӧ��ϳ�PMAA���л�������·�ߣ�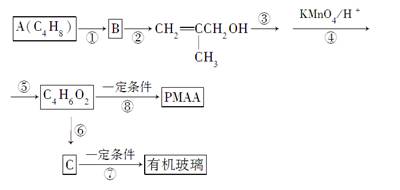
���������գ�
(1)д���ṹ��ʽ��A____________��B____________��PMAA______________��
(2)д����Ӧ���ͣ���Ӧ��________����Ӧ��______����Ӧ��________��
(3)д����Ӧ��������Ӧ��________����Ӧ��______________________��
(4)��Ӧ�ۺ͢ݵ�Ŀ����_________________________________��
(5)д����Ӧ�ߵĻ�ѧ����ʽ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
PTT�ǽ�������Ѹ�ٷ�չ���������������Ծ������ϣ������������ܣ�����Ϊ�������ϡ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�á���ϳ�·�߿����Ϊ��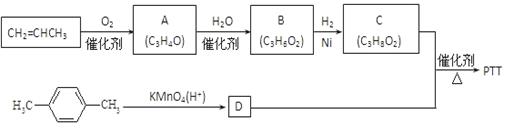
����A��B��C��Ϊ��״�����A�ܷ���������Ӧ��C�в�������1mol C���������Ʒ�Ӧ����22��4 L H2����״��������ش��������⣺
��1��A�����������ŵ�����Ϊ ��B�Ľṹ��ʽΪ ��
��2��������C��D��Ӧ����PTT�Ļ�ѧ����ʽΪ ��
��Ӧ����Ϊ ��
��3������ʽΪC4H6O��A��Ϊͬϵ���ͬ���칹���� �֡�
��4����д����CH2=CHCH3Ϊ��Ҫԭ�ϣ����Լ����ã��Ʊ�CH3CH(OH)COOH�ĺϳ�·������ͼ����ע����Ӧ��������
��֪��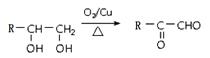
���ϳ�·�߳��õı�ʾ��ʽΪ��A B����
B����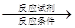 Ŀ����
Ŀ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���H��һ�ֺϳ�ҩ����м��壬H�ĺϳɷ������£�
��֪��R-CH=CH2 R-CH2-CH2OH
R-CH2-CH2OH
��ش��������⣺
��1��A����Է�������Ϊ104�ķ�������A�ķ���ʽΪ ��
��2������A��C�����л����Լ�Ϊ ��
��3��FΪһ�ȴ����˴Ź����������������շ壬��E�Ľṹ��ʽΪ ��
��4��д��B��C�Ļ�ѧ����ʽ�� ��
��5��д��D��G����H�Ļ�ѧ����ʽ�� ��
��6��D��ͬ���칹��������FeCl3��Һ������ɫ��Ӧ���ܷ���������Ӧ���� �֣�д�����к����������������շ���Ŀ���ٵĽṹ��ʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ij�����廯����A��ˮ��Һ�����ԣ���FeCl3����ɫ���ɷ�������ת������ͼ��ʾ��������IΪ��Ԫ��״�����J��ʹ��ˮ��ɫ��I��J��Ϊͬ���칹�壻K��L����ҽ�ø߷��Ӳ��ϡ�
��ش��������⡣
��1��A�Ľṹ��ʽΪ______________��
��2��д�����з�Ӧ�ķ�Ӧ���ͣ���Ӧ������________��Ӧ����Ӧ������________��Ӧ��
��3��д����Ӧ�ں͢ݵĻ�ѧ����ʽ��
��_______________________________________________________________��
��______________________________________________________________��
��4��д����������Ҫ���G��ͬ���칹��______________________________��
�ٺ��б��������ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ������ϡNaOH��Һ�У�1 mol��ͬ���칹������2 mol NaOH��Ӧ����ֻ����������һ�ȴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ƽ�����ھ��������ƣ���ṹΪ��
(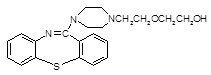 )2��
)2��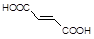 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�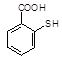

��֪������Ӧ��Ϊȡ����Ӧ������A��ϵͳ����Ϊ1,4������D2�D��ϩ��
��ش��������⣺
��1����Ӧ�ٵIJ���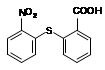 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
��2����Ӧ�۵������� ��Ӧ����Ӧ�ݵ�Ŀ���� ��
��3��д����Ӧ�ܵĻ�ѧ����ʽ ��
��4������B��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹���� �֡�
��5����֪����SH�������룭OH���ơ�
����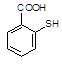 һ���������γɾۺ���Ľṹ��ʽΪ ��
һ���������γɾۺ���Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
|
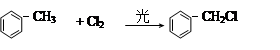 �� R��CH2��CH = CH2 + Cl2
�� R��CH2��CH = CH2 + Cl2 R��CHCl��CH = CH2 + HCl
R��CHCl��CH = CH2 + HCl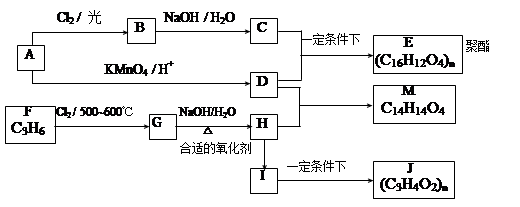
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ʊ�ż��Ⱦ��F��ҽҩ�м���Y������ͼ���£�
��֪�� ��1��
��2�� ��
��
�ش��������⣺
��1��д����Ӧ���ͣ��� ���� ��
��2��д��D��E��Ӧ�Ļ�ѧ����ʽ ��
��3��д��������A��F�Ľṹ��ʽ��A ��F ��
��4�����A��D��Ŀ���� ��
��5��д����������������C����������ͬ���칹��Ľṹ��ʽ ��
a.�DZ��Ķ�λ��ȡ����� b.�ܷ���������Ӧ
��6��������������ͼ��ƴ�X��Y�ĺϳ�·�ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������͡����Ʒ���С����ǽ⡱����һ�ֿڷ�������ҩ��������������֢��ǿ��֢����ʳ֢����ϳ�·�����£�
��1����Ӧ�ڡ��������ڻ�ԭ��Ӧ���� ������ţ���
��2��������F�к� ������̼ԭ�ӣ�������F��G��ˮ���Խ�ǿ���� ��
��3������A��ͬ���칹���У���һ���ܷ���������Ӧ�Һ˴Ź�������ͼ��4�ַ���л� �д������������Һ����������Ӧ�����ӷ���ʽ ��
��4����Ӧ�ٿ���Ϊ������ɣ���1����HCHO�Ⱥ�HN(CH3)2��Ӧ����2���������ٺ�A����ȡ����Ӧ����B����д����1����Ӧ����Ľṹ��ʽ ��
��5����֪�� ��
��
д���� ��HCHO��HN(CH3)2Ϊ�л�ԭ�ϣ��ϳ�
��HCHO��HN(CH3)2Ϊ�л�ԭ�ϣ��ϳ� �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
�ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com