
���� I����������m=CVM�����㣻
II���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
III������ƿ�������ȣ�����Һʱ�������������������������ݶ��ݵIJ�����������
IV������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺I�������������Ƶ�����m=CVM=0.622mol/L��0.5L��40g/mol=12.4g���ʴ�Ϊ��12.4��
II���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪�����������˳��Ϊ�ڢܢ٢ߢޢ�࣬�ʴ�Ϊ���ڢܢ٢ߢޢ�ࣻ
III������ƿ�������ȣ���������������ˮ���ȣ���Ҫ��������ȴ������Ȼ��ת����500mL����ƿ�У�����Һʱ������������������������ֹ��Һ����������ʱ��Ҫ��������ƿ�е�ˮ����Һ����̶���1-2cmʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�
�ʴ�Ϊ��500ml����ƿ�� ������ 1-2cm�� ��ͷ�ιܣ�
IV��A���������������ܽ�ʱδ��ȴ�����£��Ϳ�ʼת�ơ�ϴ���Լ����ݣ�����ȴ����Һ���ƫС��Ũ��ƫ�ߣ���A��ȷ��
B��ת��ʱ��С����������Һ����������ƿ�⣬��ᵼ�����ʵ���ʧ��Ũ��ƫС����B����
C������ʱ��������ƿ�̶��ߣ�����Һ���ƫ��Ũ��ƫ�ͣ���C����
D������ʱ��ˮ�����˿̶��ߣ������ý�ͷ�ι���ȥ�����ˮ���������IJ�ֹ��ˮ���������ʣ���Ũ��ƫ�ͣ���D����
E�����ƺú��ֳ���ʱ���õ�������ƽ�����������ˣ�������������������������ʵ���������Ũ��ƫ�ߣ���E��ȷ��
��ѡAE��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��ѧ�� | P-P | P-O | O�TO | P�TO |
| ����/kJ•mol-1 | a | b | c | x |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�
ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ� ���ռ乹��Ϊ�����Σ�
���ռ乹��Ϊ�����Σ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
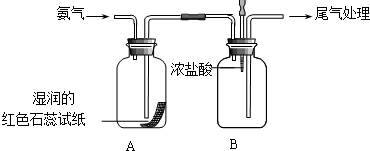 ijС��ͬѧ������ͼ��ʾװ��̽�����������ʣ�
ijС��ͬѧ������ͼ��ʾװ��̽�����������ʣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����ͼװ�ý���ʵ�飬��A��μ���B�У�
����ͼװ�ý���ʵ�飬��A��μ���B�У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ҵ��ⱥ��ʳ��ˮģ��װ�õĽṹ��ͼ��ʾ��
��ҵ��ⱥ��ʳ��ˮģ��װ�õĽṹ��ͼ��ʾ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ص�һ�������еĺ���42��ԭ�� | |
| B�� | ��������̼Ԫ�ص���������ԼΪ63.8% | |
| C�� | �����ص���Է�������Ϊ282 | |
| D�� | 0.1mol�����ص�����Ϊ28.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��a������b��ϡ���ᣨ��������a������c��Һ�У���a��b��Ӧ�����ӷ���ʽΪFe+4H++NO3-=Fe3++NO��+2H2O | |
| B�� | ��c��dΪ���壬�Ҷ���ʹ����ʯ��ˮ����ǣ��˻������ͨ����ˮ�У���ɫ��ȥ������ɫ���̵����ӷ���ʽΪSO2+Br2+2H2O�T4H++SO${\;}_{4}^{2-}$+2Br- | |
| C�� | ��c����ɫ�̼�����ζ�����壬��ˮ��Һ�������ԣ��ڱ�״�������ſ������ռ�c�����ƽ��Ħ������Ϊ20 g��mol-'1�Ļ�����������Ȫʵ�飮�������ʲ���ɢ��ʵ����ɺ�������Һ�����ʵ���Ũ��ԼΪ0�� 056 mol��L-1 | |
| D�� | ��a���������ЧӦ����Ҫ����֮һ��c��d��Ϊ���Σ��μӷ�Ӧ��a��b���ʵ���֮��Ϊ4��5����������Ӧ�����ӷ���ʽΪ4CO2+5OH-�TCO${\;}_{3}^{2-}$+3HCO${\;}_{3}^{-}$+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
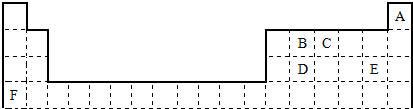
 ���Ƚ�E��F�����ּ����ӵİ뾶��С��E-���ڣ�����ڻ�С�ڣ�F+
���Ƚ�E��F�����ּ����ӵİ뾶��С��E-���ڣ�����ڻ�С�ڣ�F+�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com