



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 SiHCl3��H2)��
SiHCl3��H2)��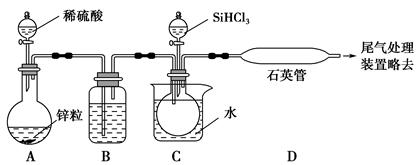
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
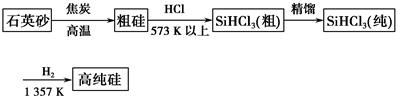
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��46.5% | B��23.3% | C��21.7% | D��35.7% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����߶�������������ʶ������ᷴӦ |
| B�����߶�������NaOH��Һ��Ӧ |
| C��CO2���ۡ��е��SiO2�� |
| D�����߶�����ˮ��Ӧ���ɶ�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SiO2����ˮ������ |
| B��CO2ͨ��ˮ�����ɵù��� |
| C����觵���Ҫ�ɷ��ǹ����� |
| D��18K������������Ʒ�����۵�ȴ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | A | B | C | D |
| ���ӳɷ� | Co2O3(������) | Cu2O | Fe2+ | PbO |
| ������ɫ�ʻ����� | ��ɫ | ��ɫ | �ֻ����� | ��ѧ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com