
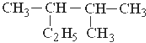 ��������
��������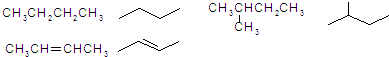
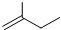
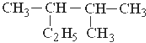 Ϊ�������������̼������5��Cԭ�ӣ�����Ϊ���飬��2��3��C����2���������л�������Ϊ��2��3-�������飬�ʴ�Ϊ��2��3-�������飻
Ϊ�������������̼������5��Cԭ�ӣ�����Ϊ���飬��2��3��C����2���������л�������Ϊ��2��3-�������飬�ʴ�Ϊ��2��3-�������飻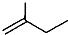 ����ʽ�ж��˺յ���̼ԭ�ӣ�ÿ��̼ԭ���γ��ĸ����ۼ���������������ʽ
����ʽ�ж��˺յ���̼ԭ�ӣ�ÿ��̼ԭ���γ��ĸ����ۼ���������������ʽ �õ����ʵķ���ʽΪC5H10���ʴ�Ϊ��C5H10��
�õ����ʵķ���ʽΪC5H10���ʴ�Ϊ��C5H10��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ���� | ����Һ�������mL�� | ���������Һ�������mL�� | |
| ��ʼ���� �ζ�ǰ | �յ���� | ||
| 1 | 20.00 | O��50 | 20.40 |
| 2 | 20.00 | 6��OO | 26.10 |
| 3 | 20.00 | 4��OO | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
6 3 |
7 3 |
23 11 |
24 12 |
14 6 |
14 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
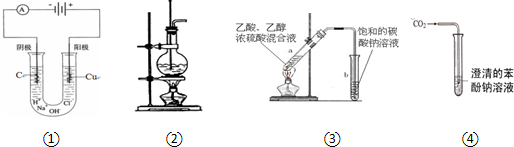
| A��װ�âٿ�����ʵ������ȡ�������� |
| B��װ�âڿ�����ʵ������ȡ������ϩ |
| C��װ�âۿ������Ʊ��������������� |
| D��װ�âܿ����ڱȽ�̼���뱽�ӵ�����ǿ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com