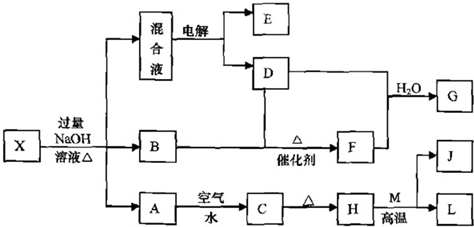
����⣺CΪ������ˮ�ĺ��ɫ���壬��CΪFe��OH��
3�����ȷֽ�ɵ�HΪFe
2O
3��H��M��Ӧ�ų��������ȣ���M��LΪ�����Ľ������ʣ�Ϊ���ȷ�Ӧ����MΪAl��LΪFe��JΪAl
2O
3��A�������ˮ��Ӧ�ɵ�Fe��OH��
3������֪AΪFe��OH��
2�����ˮ��ҺΪdz��ɫ����X�к���Fe
2+���ӣ���X�ͼӦ���ɵ�����B����B��NH
3��X���NH
4+���ӣ��ڻ��Һ�м���BaCl
2��Һ�����ɲ�����ϡ����İ�ɫ����������SO
42-���ۺ�������������֪XΪ��NH
4��
2Fe��SO
4��
2�����������Һ����������ɫ����D��E��ӦΪ���ˮ������������������D���백����������������֪DΪO
2��EΪH
2��FΪNO��NO��O
2��ˮ��Ӧ�õ�GΪHNO
3��
��1��BΪNH
3������ʽΪ
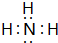
������ˮ��Һ�ʼ��ԣ���ʹʪ��ĺ�ɫʯ����ֽ������
�ʴ�Ϊ��
��ʹʪ��ĺ�ɫʯ����ֽ������
��2������������Ӧ�У��漰FeԪ�صĻ��Ϸ�ӦΪFe��OH��
2����������Fe��OH��
3����Ӧ�ķ���ʽΪ4Fe��OH��
2+O
2+2H
2O=4Fe��OH��
3��
�ʴ�Ϊ��4Fe��OH��
2+O
2+2H
2O=4Fe��OH��
3��
�ں���FeԪ�ص��û���ӦΪH��M���������ȷ�Ӧ������ʽΪFe
2O
3+2Al
2Fe+Al
2O
3��
�ʴ�Ϊ��Fe
2O
3+2Al
2Fe+Al
2O
3��
��3��Fe��������ᷴӦ�ķ���ʽΪFe+4H
++NO
3-=Fe
3++NO��+2H
2O��
�ʴ�Ϊ��Fe+4H
++NO
3-=Fe
3++NO��+2H
2O��
��4���������ƶϿ�֪������X�Ļ�ѧʽΪ��NH
4��
2Fe��SO
4��
2��
�ʴ�Ϊ����NH
4��
2Fe��SO
4��
2��
��5����ȡWg��NH
4��
2Fe��SO
4��
2?nH
2O��������ˮ�У�����100mL��Һ�����õζ���ȡ��10.00mL����Cmol?L
-l������KMnO
4��Һ���еζ����ζ��յ�ʱ����������KMnO
4��ҺVmL����n��MnO
4-��=n��KMnO
4��=0.001CV mol����
5Fe
2++MnO
4-+8H
+=5Fe
3++Mn
2++4H
2O
5 1
0.005CV mol 0.001CV mol
������X��Һ�����ʵ����ʵ���Ũ��=
=0.5CV mol/L��
��0.005CV mol��
����18n+284��g/mol=Wg�����n=
��
�ʴ�Ϊ��0.5CV��
��

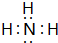 ������ˮ��Һ�ʼ��ԣ���ʹʪ��ĺ�ɫʯ����ֽ������
������ˮ��Һ�ʼ��ԣ���ʹʪ��ĺ�ɫʯ����ֽ������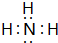 ��ʹʪ��ĺ�ɫʯ����ֽ������
��ʹʪ��ĺ�ɫʯ����ֽ������