
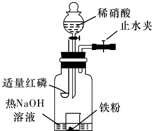
 ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽����
ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽����| 0.3��6��3 |
| 8 |
| 0.3��6��56 |
| 4 |


 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽����
a.����ƿ��ע��������NaOH��Һ����ʢ��a g�����۵�С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е���300mL 6mol/L��ϡ���ᣬ����ǡ����ȫ�ܽ⡣
(һ)�������ɷ�̽����
(1)ȼ�պ���Ŀ���ǡ���
(2)Ϊ֤���������ΪNO������c��ȱ�ٵ�һ����Ҫ��������������������
(��)��������Ԫ�ؼ�̬̽����
(1)����������裺
����1������ֻ�У�3������
����2��������������������������������������������
����3��������������������������������������������
(2)���ʵ�飺�ֱ�ȡС�ձ��з�Ӧ����Һװ����֧�Թܼס��ң��ڼ��Թ��еμ����Ը��������Һ�������Թ��еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ�������������������������������������������������������1��ȷ��
��������Ϊ�������������������������������������������������������2��ȷ��
��������Ϊ�������������������������������������������������������3��ȷ��
(3)aֵ��ΧΪ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡʮ��У����������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ������
ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽����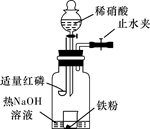
a.����ƿ��ע��������NaOH��Һ����ʢ��a g�����۵�С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е���300mL 6mol/L��ϡ���ᣬ����ǡ����ȫ�ܽ⡣
(һ)�������ɷ�̽����
(1)ȼ�պ���Ŀ���ǡ���
(2)Ϊ֤���������ΪNO������c��ȱ�ٵ�һ����Ҫ��������������������
(��)��������Ԫ�ؼ�̬̽����
(1)����������裺
����1������ֻ�У�3������
����2��������������������������������������������
����3��������������������������������������������
(2)���ʵ�飺�ֱ�ȡС�ձ��з�Ӧ����Һװ����֧�Թܼס��ң��ڼ��Թ��еμ����Ը��������Һ�������Թ��еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ�������������������������������������������������������1��ȷ��
��������Ϊ�������������������������������������������������� �����2��ȷ��
�����2��ȷ��
��������Ϊ�������������������������������������������������������3��ȷ��
(3)aֵ��ΧΪ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ��ɽ�и�����ѧģ���Ծ���ʮ�� ���ͣ�ʵ����
��16�֣�ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽���������ԭ������Fe��56��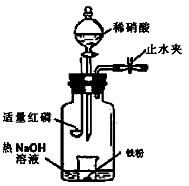
a������ƿ��ע��������NaOH��Һ����ʢ��a g�����۵�С�ձ�����ƿ�С�
b���ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c�������׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е���200mL 6mol/L��ϡ���ᣬ����ǡ����ȫ�ܽ⡣
��һ���������ɷ�̽��
��ʵ��ǰ��μ���װ�õ������� ��
��ȼ�պ���Ŀ���� ��
��Ϊ֤���������ΪNO������c��ȱ�ٵ�һ����Ҫ������___________________��
��������������Ԫ�ؼ�̬̽����
��1������������裺
����1������ֻ��+3������
����2�� ��
����3�� ��
��2�����ʵ�飺�ֱ�ȡС�ձ��з�Ӧ����Һװ����֧�Թܼס��ң��ڼ��Թ��еμ����Ը��������Һ�������Թ��еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ�� �������1��ȷ��
��������Ϊ�� �������2��ȷ��
��������Ϊ�� �������3��ȷ��
��3��aֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡʮ��У����������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ������
ijͬѧ��ͼʾװ�ý�������ϡ���ᷴӦ��ʵ�鲢������ص�ʵ��̽����
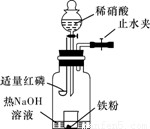
a.����ƿ��ע��������NaOH��Һ����ʢ��a g�����۵�С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е���300mL 6mol/L��ϡ���ᣬ����ǡ����ȫ�ܽ⡣
(һ)�������ɷ�̽����
(1)ȼ�պ���Ŀ���ǡ���
(2)Ϊ֤���������ΪNO������c��ȱ�ٵ�һ����Ҫ��������������������
(��)��������Ԫ�ؼ�̬̽����
(1)����������裺
����1������ֻ�У�3������
����2��������������������������������������������
����3��������������������������������������������
(2)���ʵ�飺�ֱ�ȡС�ձ��з�Ӧ����Һװ����֧�Թܼס��ң��ڼ��Թ��еμ����Ը��������Һ�������Թ��еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ�������������������������������������������������������1��ȷ��
��������Ϊ�������������������������������������������������������2��ȷ��
��������Ϊ�������������������������������������������������������3��ȷ��
(3)aֵ��ΧΪ������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com