
����Ŀ����1����ѧ�ҿ���ͨ��__________������̫�������д�������Ԫ�أ�д����̬Feԭ�ӵļ۵����Ų�ͼ__________________���ӽṹ�Ϸ���Fe3+��Fe2+�ȶ���ԭ��________________________��
��2��SCN-���������Fe3+�Ĵ��ڣ�SCN-����ԭ�ӵ��ӻ���ʽΪ_________������Ԫ�ص縺���ɴ�С��˳��Ϊ__________________��
��3���黯���Ե������뵼�����ƣ��۵�Ϊ1230�棬���пռ���״�ṹ����֪���������黯������ͬ�־������͡������־����۵�ϸߵ���_________(�ѧʽ)����������________________________��
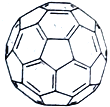
��4��C60������������ϩ��������60��̼ԭ����ɵ���״���ӣ���ͼΪC60�ṹͼ��һ��C60��������Ԫ������ĿΪ____________����C60������C60���ӵ���λ��Ϊ___________����֪C60���ӵ����� �뾶Ϊa nm����C60�������=_________g/cm3��(��NA��ʾ�����ӵ�������д������ʽ)
���𰸡� ԭ�ӹ��� ![]() Fe3+�۵����Ų�ʽΪ3d5��Ϊ�����ṹ����Fe2+�۵����Ų�ʽΪ3d6 sp N>S>C BN ���־����Ϊԭ�Ӿ��壬N ��B ԭ�Ӱ뾶��С�����ܽϴ��۷е���� 20 12
Fe3+�۵����Ų�ʽΪ3d5��Ϊ�����ṹ����Fe2+�۵����Ų�ʽΪ3d6 sp N>S>C BN ���־����Ϊԭ�Ӿ��壬N ��B ԭ�Ӱ뾶��С�����ܽϴ��۷е���� 20 12 ![]() ��1021
��1021
��������������������⿼��ԭ�ӹ��ס��۵����Ų�ͼ����д���ӻ���ʽ���жϡ��縺�Դ�С�ıȽϡ������۵�ߵ͵ıȽϡ������ķ����ͼ��㡣
��1������Ԫ�س���ԭ�ӹ��ף���ѧ�ҿ���ͨ��ԭ�ӹ�������̫�����ڴ�������Ԫ�ء�Feԭ�Ӻ�����26�����ӣ����ݹ���ԭ������̬Feԭ�ӵĺ�������Ų�ʽΪ[Ar]3d64s2����̬Feԭ�ӵļ۵����Ų�ʽΪ3d64s2����̬Feԭ�ӵļ۵����Ų�ͼΪ��![]() ��Fe3+�ļ۵����Ų�ʽΪ3d5��Ϊ�����ṹ���ȶ���Fe2+�ļ۵����Ų�ʽΪ3d6������Fe3+��Fe2+�ȶ���
��Fe3+�ļ۵����Ų�ʽΪ3d5��Ϊ�����ṹ���ȶ���Fe2+�ļ۵����Ų�ʽΪ3d6������Fe3+��Fe2+�ȶ���
��2��SCN-��CO2��Ϊ�ȵ����壬CO2������ԭ�ӵ��ӻ���ʽΪsp�ӻ�����SCN-������ԭ�ӵ��ӻ���ʽҲΪsp�ӻ������ݵ縺��Խ��Ԫ�صķǽ�����Խǿ���ǽ�������N![]() S
S![]() C��S��C��N����Ԫ�ص縺���ɴ�С��˳���ǣ�N
C��S��C��N����Ԫ�ص縺���ɴ�С��˳���ǣ�N![]() S
S![]() C��
C��
��3���黯���۵�ϸߣ����пռ���״�ṹ���黯������ԭ�Ӿ��壻���������黯������ͬ�־������ͣ�������Ҳ����ԭ�Ӿ������������ԭ�Ӱ뾶С���顢�ص�ԭ�Ӱ뾶���������й��ۼ��ļ��ܴ����黯���й��ۼ��ļ��ܣ����־������۵�ϸߵĵ����𣬵�����Ļ�ѧʽΪBN��
��4������ͼʾ��ÿ��̼ԭ���γ�3��̼̼����2��̼̼������1��̼̼˫������ÿ����Ԫ����Χ��5����Ԫ����ÿ����Ԫ����Χ��3����Ԫ����3����Ԫ������Ԫ������Ԫ���ĸ�����Ϊ3:5������Ԫ������Ԫ���ֱ���x��y������x��y=3:5��5x+6y=3![]() 60�����x=12��y=20��һ��C60��������Ԫ������ĿΪ20����C60������C60���Ӳ�ȡ�����ܶѻ���1��C60������Χ�Ⱦ��������C60������12����C60���ӵ���λ��Ϊ12��C60����Ϊ�����������ܶѻ���������̯������1�������к�C60��8
60�����x=12��y=20��һ��C60��������Ԫ������ĿΪ20����C60������C60���Ӳ�ȡ�����ܶѻ���1��C60������Χ�Ⱦ��������C60������12����C60���ӵ���λ��Ϊ12��C60����Ϊ�����������ܶѻ���������̯������1�������к�C60��8![]() +6
+6![]() =4����C60���ӵ�����뾶Ϊanm�������ı߳�Ϊ2
=4����C60���ӵ�����뾶Ϊanm�������ı߳�Ϊ2![]() anm��1�����������Ϊ��2
anm��1�������������2![]() anm��3=16
anm��3=16![]() a3
a3![]() 10-21cm3��1mol��������Ϊ16
10-21cm3��1mol��������Ϊ16![]() a3
a3![]() 10-21cm3
10-21cm3![]() 4
4![]() NA=4
NA=4![]() a3NA
a3NA![]() 10-21cm3��1molC60���������Ϊ720g��C60�������=720g
10-21cm3��1molC60���������Ϊ720g��C60�������=720g![]() ��4
��4![]() a3NA
a3NA![]() 10-21cm3��=
10-21cm3��=![]() 1021g/cm3��
1021g/cm3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��40��ʱ���ڰ�-ˮ��ϵ�в���ͨ��CO2���������ӱ仯������ͼ��ʾ������˵������ȷ����
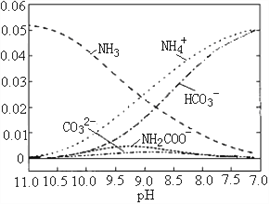
A. ��8.5<pH<10.5ʱ�������м���NH2COO��������
B. ��pH��9.5ʱ����Һ�д��ڹ�ϵ��c(NH4+)��c(HCO3��)��c(NH2COO��)��c(CO32��)
C. ��pH��9.0ʱ����Һ�д��ڹ�ϵ��c(NH4+)��c(H+)��2c(CO32��)��c(HCO3��)��c(NH2COO��)��c(OH��)
D. ����CO2��ͨ�룬![]() ���ϼ�С
���ϼ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. ������Ư�۳���������ˮ�ľ���������������ԭ����ͬ
B. �����£�ͬŨ�ȵ�Na2S��NaHS��Һ��ȣ�Na2S��Һ��pHС
C. �����ʵ���Ũ�ȵ�NH4Cl��Һ��NH4HSO4��Һ�����ߵ�c(NH4��)��
D. FeCl3��KSCN��Ӧ�ﵽƽ��ʱ������KCl��Һ������Һ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�飺�ٳ�ȥ����ֲ�����е�ˮ���ڶԵ�ˮ�еĵ����Ũ�����۶�30%�ľƾ���Һ�еľƾ������ᴿ������ʵ����õ���ȷ���������ǣ� ��
A.��Һ����ȡ������B.��ȡ������Һ
C.��Һ��������ȡD.������ȡ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������м��ܵ��磬������ǿ����ʵ�һ�������ǣ� ��
A.ʯī��������Һ��ʳ�ξ���
B.����״̬��KOH������״̬��NaCl
C.ϡH2SO4��NaOH��Һ����HNO3
D.Һ����ʯ��ˮ��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�������5.6L��CO2����Ϊ _____g,���к���______�����ӣ�����_____��ԭ�ӣ�
��2��������Ϊm g�� HCl��NH3��CO2��O2�������壬����������Ŀ���ٵ���_________�����������_____���ܶ���С����_______������ͬ�¶Ⱥ�ѹǿ�����£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊ������ʵ�鰲ȫҪ����ǣ� ��
A.Ϩ��ƾ���ʱ���õ�ñ����
B.��ȼ����ǰ���Ƚ����鴿����
C.����ϡ����ʱ����Ũ���Ỻ������ˮ�в����Ͻ���
D.��������й©ʱ��Ѹ���뿪�ֳ����������ʹ�ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᡣ
�����Ʊ�������ͻ���δ��Ӧ�ļױ�
��Ӧԭ����
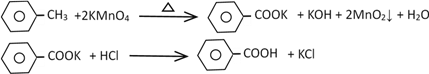
��1����һ�����ļױ���KMnO4��Һ��100����Ӧһ��ʱ���ֹͣ��Ӧ��
��2�������˷�Ӧ�����õ���Һ�����������õ��л����ˮ�㣻
��3�������л����м���ˮNa2SO4�����ˣ����������õ���ɫҺ��A��
��4������ˮ�����Ũ�����ữ������Ũ������ȴ�����ˣ��õ���ɫ����B��
��֪��
��Է������� | �۵� | �е� | �ܶ� | �ܽ�� | |
�ױ� | 92 | ��95�� | 110.8�� | 0.8669g��mL��1 | ������ˮ |
������ | 122 | 122.4�� | 249�� | 1.2659 g��mL��1 | 0.3g (25��ʱ) 6.9g (95��ʱ) |
��1�����������õIJ����������ձ���______________��������Ϊ________________��
��2����3���м�����ˮNa2SO4��Ŀ����_____________________����ɫҺ��A��_______��
�����ᴿ�ֱ�����
��ͬѧ�����ؽᾧ�ķ����Եõ���B�����ᴿ���ؽᾧ�Ĺ��̣������ܽ�����ȳ��ˡ���ȴ�ᾧ�����ˡ�ϴ�ӡ�������������ᡣ��ע������װ����ͼ��ʾ����Ҫ������A����©����B����ƿ�������õȣ�
��3����ɫ����B�е�������____________��
��4�����ȳ��˵õ�����Һ������ȴ���Խᾧ�������ı����ᾧ�壬Ϊ�˵õ�����ı����ᣬ�Dz����¶�Խ��Խ�ò�˵������______����ǡ����ǡ���������__________________________________________________________��
��5��ʹ�ó���װ�ñ���ͨ����װ�õ��ŵ���_______________________________________��
��6��ʵ����ȡ�ױ�10.0 mL���Ƶñ�����8.0g������ʵ���б�����IJ���Ϊ____________�����������С�����һλ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�á�
��1��д���������������ƣ�a��__________�� b��___________��
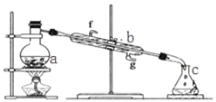
��2��ʵ������У���Ҫͨ��ˮ��ͼ�еĽ�ˮ������_______������ͼ����ĸ����
��3��������װ�÷������ᣨ�е�118�棩�������������е�77�棩�Ļ�����ȱ�ٵ�������_______��
II������NaOH��������0.1 mol/L NaOH��Һ480mL���ݴ˻ش��������⣺
��4����������������Һ��Ҫ�õ��������������ձ�������������ͷ�ιܺ�______��
��5��ʵ��ʱ��Ҫ������������_______g��
��6������0.1 mol/L NaOH��Һ��ʵ���У�����������²������ᵼ��������Һ��Ũ��ƫ�����_______����д��ĸ����
A�������������ƹ���ʱ����ŷ��� B��δϴ���ܽ�NaOH���ձ�
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ��
D������ƿδ���T����������Һ E������ʱ���ӿ̶���
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com