
���� ��1������������ʵ�ǰ��л����ɺܶ�С�飨�й��ɵģ����ո������Ŵ�����һ���е㾭���Ҳ�ܴ�С���ֿ����������ʴ�ź�����Щ�����ţ������кܶͬ�ķ��������֣����������Ǹ����Ǹ��л���ķ�������
�ڸ����л���ȼ��ʱ���л����е�̼ȫ��ת��Ϊ������̼���ɶ�����̼�����������������Cԭ�Ӹ������л����е���ȫ��ת��Ϊˮ����ˮ�����������Կ��������Hԭ�Ӹ�����Ȼ���ж��Ƿ�����ԭ�ӣ����ȷ��M�ķ���ʽ��
�۸�����������Ȳ��һ����������ȡMд����Ӧ�Ļ�ѧ����ʽ�����������Ը��������Һ����ˮ�ж�M�������Ƿ���̼̼˫����̼̼�������Ӷ�ȷ����ṹ��
��� �⣺��1������������ʵ�ǰ��л����ɺܶ�С�飬���кܶͬ�ķ��������֣����������Ǹ����Ǹ��л���ķ��������ʿ�����ȷ���л�����Է������ķ�����b��
�ʴ�Ϊ��b��
�ڶ�����̼����Ϊ26.4g��n��CO2��=$\frac{26.4g}{44g/mol}$=0.6mol��n��C��=n��CO2��=0.6mol��ˮ������Ϊ5.4g��n��H2O��=$\frac{5.4g}{18g/mol}$=0.3mol��n��H��=2n��H2O��=2��0.3mol=0.6mol������C��Hԭ�ӵ�������Ϊ��12g/mol��0.6mol+1g/mol��0.6mol=7.8g��˵��M�����в�����Oԭ�ӣ�
7.8gM�����ʵ���Ϊ��$\frac{7.8g}{78g/mol}$=0.1mol����1molM�����к���6��C��6��Hԭ�ӣ�����M�ķ���ʽΪC6H6��Ϊ����
�ʴ�Ϊ��C6H6��
����������Ȳ��һ����������ȡM���÷�Ӧ�Ļ�ѧ����ʽΪ��3CH��CH$\stackrel{����}{��}$C6H6��
ȷ��C6H6�Ľṹ����Ҫ�ж���������Ƿ���̼̼˫����˫������������ˮ�����Ը��������Һ���𣬹�AB��ȷ��
�ʴ�Ϊ��3CH��CH$\stackrel{����}{��}$C6H6��AB��
���� ���⿼���л��ƶϣ���Ŀ�ѶȲ�����ȷ�����л���Ľṹ������Ϊ���ؼ���ע������ȷ���л������ʽ���ṹ��ʽ�ķ�����������ػ���֪ʶ�Ŀ��飬���������ѧ���ķ���������������������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | NaHSO4���������Ӿ��� | |
| B�� | NaHSO4�����������Ӻ������ӵĸ�����2��1 | |
| C�� | NaHSO4�����ۻ�ʱ�ƻ��������Ӽ����ۼ� | |
| D�� | NaHSO4��������ˮʱ���ƻ����Ӽ����ƻ����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | x+y=7 | B�� | x+y=9 | C�� | x+y=5 | D�� | x+y=11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������NaOH��Һ��Ӧ�����ӷ���ʽΪ��H++OH-�TH2O | |
| B�� | ���������Ҵ���Ӧ�Ļ�ѧ����ʽΪ��2Na+2CH3CH2OH��2CH3CH2ONa+H2�� | |
| C�� | ������ϩ�Ľṹ��ʽΪ��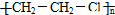 | |
| D�� | �Ȼ�淋ĵ���ʽΪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��c��HCO3-��=0.1 mol•L-1����Һ�У�NH4+��AlO2-��Cl-��NO3- | |
| B�� | ����ˮ�������c��H+��=1��10-12 mol•L-1����Һ�У�Fe2+��ClO-��Na+��SO42- | |
| C�� | KW/c��H+��=10-10 mol•L-1����Һ�У�Na+��HCO3-��Cl-��K+ | |
| D�� | ��ʹ��ɫʯ����ֽ��������Һ�У�SO32-��CO32-��Na+��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
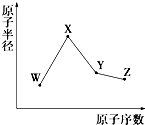 W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵����ȷ���ǣ�������
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�������ͬ����Ԫ������ǿ������˵����ȷ���ǣ�������| A�� | ��Ӧ�����Ӱ뾶��X��W | |
| B�� | ��Ӧ��̬�⻯����ȶ��ԣ�Y��Z | |
| C�� | ������XZW�Ⱥ����Ӽ����ֺ����ۼ�������������� | |
| D�� | Y���������Z���⻯�������X������������Ӧ��ˮ���ﷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������������ЧӦ�������⻯ѧ���������γ��뵪���������� | |
| B�� | �ơ������ⶼ�������ڱ������Ԫ�� | |
| C�� | ���л��������ܻ�������ͨ�����˳�ȥ | |
| D�� | �Ǹ�ɽʱ��ɹ��Ϊ�˷�ֹǿ����������Ƥ�������ʱ����˱��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com