
 дкtЁцЪБЃЌЯђШнЛ§ЮЊ2LЕФУмБеШнЦїМзжаМгШы1mol AЦјЬхЁЂ3mol BЦјЬхМАЩйСПЙЬЬхДпЛЏМСЃЌЗЂЩњЯТСаЗДгІЃКAЃЈgЃЉ+3BЃЈgЃЉ?2CЃЈgЃЉЃЛЁїHЃМ0ЃЌ10minКѓИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУЦјЬхCЕФЬхЛ§ЗжЪ§ЮЊ25%ЃЎЧыЬюПеЃК
дкtЁцЪБЃЌЯђШнЛ§ЮЊ2LЕФУмБеШнЦїМзжаМгШы1mol AЦјЬхЁЂ3mol BЦјЬхМАЩйСПЙЬЬхДпЛЏМСЃЌЗЂЩњЯТСаЗДгІЃКAЃЈgЃЉ+3BЃЈgЃЉ?2CЃЈgЃЉЃЛЁїHЃМ0ЃЌ10minКѓИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУЦјЬхCЕФЬхЛ§ЗжЪ§ЮЊ25%ЃЎЧыЬюПеЃК| 2x |
| 4-2x |
| ||
| 10min |
| 0.4mol |
| 1mol |



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
(14 Зж)дкT1ЁцЪБЃЌЯђШнЛ§ЮЊ2 L ЕФУмБеШнЦїМзжаМгШЫ1mol N1ЁЂ3mol H2МАЩйСПЙЬЬхДп ЛЏМСЃЌЗЂЩњЗДгІN2(g) + 3H2(g) ![]() 2NH3(g)ЃЛЁїHЃМ0ЃЌ10minЪБИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУNH3 ЕФЬхЛ§ЗжЪ§ЮЊ25% ЁЃ
2NH3(g)ЃЛЁїHЃМ0ЃЌ10minЪБИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУNH3 ЕФЬхЛ§ЗжЪ§ЮЊ25% ЁЃ
(1) ИУЗДгІдк0 ~l0min ЪБМфФкH2ЕФЦНОљЗДгІЫйТЪЮЊ______ЃЌN2ЕФзЊЛЏТЪЮЊ______ЁЃ
 (2)дкT1ЁцЪБЃЌШєЦ№ЪМЪБдкШнЦїМзжаМгШы0.5mol N2ЁЂ1.5mol H2ЁЂ0.5mol NH3 ЃЌдђДяЕНЦНКтЪБNH3ЕФЬхЛ§ЗжЪ§______ (ЬюбЁЯюзжФИ) ЁЃ
(2)дкT1ЁцЪБЃЌШєЦ№ЪМЪБдкШнЦїМзжаМгШы0.5mol N2ЁЂ1.5mol H2ЁЂ0.5mol NH3 ЃЌдђДяЕНЦНКтЪБNH3ЕФЬхЛ§ЗжЪ§______ (ЬюбЁЯюзжФИ) ЁЃ
a.Дѓгк25% b.ЕШгк25% c.аЁгк25%
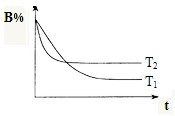
(3)гвЭМЪЧдкT1ЁцЪБУмБеШнЦїМзжаH2ЕФЬхЛ§ЗжЪ§ЫцЪБМфtЕФБфЛЏЧњЯпЃЌЧыдкИУЭМжаВЙЛГіИУЗДгІдкT2ЁцЃЈT1 ЃОT2ЃЉЪБH2ЕФЬхЛ§ЗжЪ§ЫцЪБМфtЕФБфЛЏЧњЯпЁЃ
(4)ШєБЃГжT1ЁцЃЌдкЬхЛ§вВЮЊ2 L ЕФУмБеШнЦїввжаЭЈШывЛЖЈСПЕФN2ЁЂH2ЁЂNH3ЃЌгћЪЙЦНКтЪБШнЦїввжаИїЮяжЪЕФЮяжЪЕФСПгыШнЦїМзжаЭъШЋЯрЭЌЃЌЧвЦ№ЪМЪБЗДгІЯђе§ЗДгІЗНЯђНјааЃЌдђЭЈШыH2ЕФЮяжЪЕФСПxЕФШЁжЕЗЖЮЇЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2010ФъЪЏМвзЏЪаИпжаБЯвЕАрИДЯАНЬбЇжЪСПМьВтЖўРэПЦзлКЯФмСІВтЪдЛЏбЇЪдЬт ЬтаЭЃКЬюПеЬт
дкT1ЁцЪБЃЌЯђШнЛ§ЮЊ2 L ЕФУмБеШнЦїМзжаМгШЫ1mol N1ЁЂ3mol H2МАЩйСПЙЬЬхДп ЛЏМСЃЌЗЂЩњЗДгІN2(g) + 3H2(g)  2NH3(g)ЃЛЁїHЃМ0ЃЌ10minЪБИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУNH3 ЕФЬхЛ§ЗжЪ§ЮЊ25% ЁЃ
2NH3(g)ЃЛЁїHЃМ0ЃЌ10minЪБИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУNH3 ЕФЬхЛ§ЗжЪ§ЮЊ25% ЁЃ
(1) ИУЗДгІдк0 ~l0min ЪБМфФкH2ЕФЦНОљЗДгІЫйТЪЮЊ______ЃЌN2ЕФзЊЛЏТЪЮЊ______ЁЃ (2)дкT1ЁцЪБЃЌШєЦ№ЪМЪБдкШнЦїМзжаМгШы0.5mol N2ЁЂ1.5mol H2ЁЂ0.5mol NH3 ЃЌдђДяЕНЦНКтЪБNH3ЕФЬхЛ§ЗжЪ§______ (ЬюбЁЯюзжФИ) ЁЃ
(2)дкT1ЁцЪБЃЌШєЦ№ЪМЪБдкШнЦїМзжаМгШы0.5mol N2ЁЂ1.5mol H2ЁЂ0.5mol NH3 ЃЌдђДяЕНЦНКтЪБNH3ЕФЬхЛ§ЗжЪ§______ (ЬюбЁЯюзжФИ) ЁЃ
a.Дѓгк25% b.ЕШгк25% c.аЁгк25%
(3)гвЭМЪЧдкT1ЁцЪБУмБеШнЦїМзжаH2ЕФЬхЛ§ЗжЪ§ЫцЪБМфtЕФБфЛЏЧњЯпЃЌЧыдкИУЭМжаВЙЛГіИУЗДгІдкT2ЁцЃЈT1ЃОT2ЃЉЪБH2ЕФЬхЛ§ЗжЪ§ЫцЪБМфtЕФБфЛЏЧњЯпЁЃ
(4)ШєБЃГжT1ЁцЃЌдкЬхЛ§вВЮЊ2 L ЕФУмБеШнЦїввжаЭЈШывЛЖЈСПЕФN2ЁЂH2ЁЂNH3ЃЌгћЪЙЦНКтЪБШнЦїввжаИїЮяжЪЕФЮяжЪЕФСПгыШнЦїМзжаЭъШЋЯрЭЌЃЌЧвЦ№ЪМЪБЗДгІЯђе§ЗДгІЗНЯђНјааЃЌдђЭЈШыH2ЕФЮяжЪЕФСПxЕФШЁжЕЗЖЮЇЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃККўББЪЁФЃФтЬт ЬтаЭЃКЬюПеЬт
 2C(g)ЃЛЁїH
2C(g)ЃЛЁїH 
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
дкT1ЁцЪБЃЌЯђШнЛ§ЮЊ2 L ЕФУмБеШнЦїМзжаМгШЫ1mol N1ЁЂ3mol H2МАЩйСПЙЬЬхДп ЛЏМСЃЌЗЂЩњЗДгІN2(g) + 3H2(g) ![]() 2NH3(g)ЃЛЁїHЃМ0ЃЌ10minЪБИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУNH3 ЕФЬхЛ§ЗжЪ§ЮЊ25% ЁЃ
2NH3(g)ЃЛЁїHЃМ0ЃЌ10minЪБИїЮяжЪЕФХЈЖШВЛдйБфЛЏЃЌВтЕУNH3 ЕФЬхЛ§ЗжЪ§ЮЊ25% ЁЃ
(1) ИУЗДгІдк0 ~l0min ЪБМфФкH2ЕФЦНОљЗДгІЫйТЪЮЊ______ЃЌN2ЕФзЊЛЏТЪЮЊ______ЁЃ
 (2)дкT1ЁцЪБЃЌШєЦ№ЪМЪБдкШнЦїМзжаМгШы0.5mol N2ЁЂ1.5mol H2ЁЂ0.5mol NH3 ЃЌдђДяЕНЦНКтЪБNH3ЕФЬхЛ§ЗжЪ§______ (ЬюбЁЯюзжФИ) ЁЃ
(2)дкT1ЁцЪБЃЌШєЦ№ЪМЪБдкШнЦїМзжаМгШы0.5mol N2ЁЂ1.5mol H2ЁЂ0.5mol NH3 ЃЌдђДяЕНЦНКтЪБNH3ЕФЬхЛ§ЗжЪ§______ (ЬюбЁЯюзжФИ) ЁЃ
a.Дѓгк25% b.ЕШгк25% c.аЁгк25%
(3)гвЭМЪЧдкT1ЁцЪБУмБеШнЦїМзжаH2ЕФЬхЛ§ЗжЪ§ЫцЪБМфtЕФБфЛЏЧњЯпЃЌЧыдкИУЭМжаВЙЛГіИУЗДгІдкT2ЁцЃЈT1 ЃОT2ЃЉЪБH2ЕФЬхЛ§ЗжЪ§ЫцЪБМфtЕФБфЛЏЧњЯпЁЃ
(4)ШєБЃГжT1ЁцЃЌдкЬхЛ§вВЮЊ2 L ЕФУмБеШнЦїввжаЭЈШывЛЖЈСПЕФN2ЁЂH2ЁЂNH3ЃЌгћЪЙЦНКтЪБШнЦїввжаИїЮяжЪЕФЮяжЪЕФСПгыШнЦїМзжаЭъШЋЯрЭЌЃЌЧвЦ№ЪМЪБЗДгІЯђе§ЗДгІЗНЯђНјааЃЌдђЭЈШыH2ЕФЮяжЪЕФСПxЕФШЁжЕЗЖЮЇЪЧ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com