
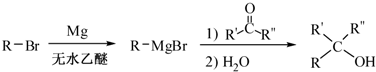
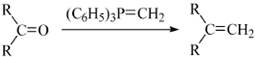


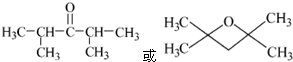


 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C�TCH2
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C�TCH2| HBr |
| NaOH��Һ |
| �� |
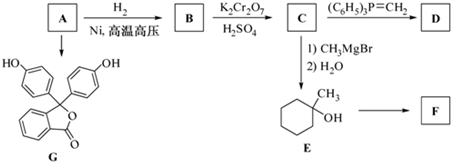
 ��A��һ�������º����������ӳɷ�Ӧ����B��B�Ľṹ��ʽΪ��
��A��һ�������º����������ӳɷ�Ӧ����B��B�Ľṹ��ʽΪ�� ��B���ظ������Һ��������C��C�Ľṹ��ʽΪ��
��B���ظ������Һ��������C��C�Ľṹ��ʽΪ�� ��C��Ӧ����D����������Ϣ֪��D�Ľṹ��ʽΪ��
��C��Ӧ����D����������Ϣ֪��D�Ľṹ��ʽΪ�� ��C����һϵ�з�Ӧ����E��E��Ӧ����F��F��D��Ϊͬ���칹�壬����E������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��
��C����һϵ�з�Ӧ����E��E��Ӧ����F��F��D��Ϊͬ���칹�壬����E������ȥ��Ӧ����F����F�Ľṹ��ʽΪ�� ��
�� ��A��һ�������º����������ӳɷ�Ӧ����B��B�Ľṹ��ʽΪ��
��A��һ�������º����������ӳɷ�Ӧ����B��B�Ľṹ��ʽΪ�� ��B���ظ������Һ��������C��C�Ľṹ��ʽΪ��
��B���ظ������Һ��������C��C�Ľṹ��ʽΪ�� ��C��Ӧ����D����������Ϣ֪��D�Ľṹ��ʽΪ��
��C��Ӧ����D����������Ϣ֪��D�Ľṹ��ʽΪ�� ��C����һϵ�з�Ӧ����E��E��Ӧ����F��F��D��Ϊͬ���칹�壬����E������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��
��C����һϵ�з�Ӧ����E��E��Ӧ����F��F��D��Ϊͬ���칹�壬����E������ȥ��Ӧ����F����F�Ľṹ��ʽΪ�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��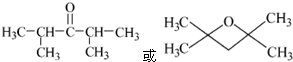 ��
��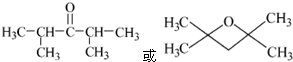 ��
�� ���ڼ��ȡ�Ũ����������������E������ȥ��Ӧ����F�����Է�ӦE��F�Ļ�ѧ����ʽΪ��
���ڼ��ȡ�Ũ����������������E������ȥ��Ӧ����F�����Է�ӦE��F�Ļ�ѧ����ʽΪ�� ��
�� ��
�� ��
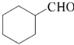
�� �ͼ�ȩ��ˮ��Ӧ����
�ͼ�ȩ��ˮ��Ӧ���� ��
�� ��ͭ�����������������±�������������
��ͭ�����������������±������������� ��
�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?���ն�ģ��ͼ��ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ��������װ�ã���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼���װ�ã��й����ݼ�����
��2013?���ն�ģ��ͼ��ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ��������װ�ã���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼���װ�ã��й����ݼ�����| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫ Һ�� |
��ɫ Һ�� |
�����ɫ Һ�� |
| �ܶ�/g?cm-3 | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?���գ�������������FeC6H6O7����һ�������յĸ�Ч���Ƽ��������̷���FeSO4?7H2O��ͨ�����з�Ӧ�Ʊ���FeSO4+Na2CO3�TFeCO3��+Na2SO4 FeCO3+C6H8O7�TFeC6H6O7+CO2��+H2O �±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol?L-1���㣩��
��2013?���գ�������������FeC6H6O7����һ�������յĸ�Ч���Ƽ��������̷���FeSO4?7H2O��ͨ�����з�Ӧ�Ʊ���FeSO4+Na2CO3�TFeCO3��+Na2SO4 FeCO3+C6H8O7�TFeC6H6O7+CO2��+H2O �±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol?L-1���㣩��| �������� | ��ʼ������pH | ������ȫ��pH |
| Fe3+ | 1.1 | 3.2 |
| Al3+ | 3.0 | 5.0 |
| Fe2+ | 5.8 | 8.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
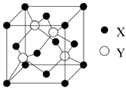 ��2013?���գ�A��[���ʽṹ������]
��2013?���գ�A��[���ʽṹ������]�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
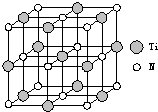 ��2013?������ģ����5-��������
��2013?������ģ����5-��������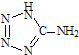 ���м������Ga���õ�������һ���������巢������������������ȫ���ң�
���м������Ga���õ�������һ���������巢������������������ȫ���ң��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
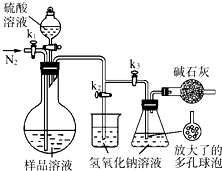 ��2013?������ģ��̼����-��������Ӻ��aNa2CO3?bH2O2������Ư�ס�ɱ�����ã�ʵ�����á����������Ʊ������ʵ�ʵ�鲽�����£�
��2013?������ģ��̼����-��������Ӻ��aNa2CO3?bH2O2������Ư�ס�ɱ�����ã�ʵ�����á����������Ʊ������ʵ�ʵ�鲽�����£��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com