
��2009?�㶫���������ˮ�ⷴӦ����ʽΪ��
HCOOCH
3��l��+H
2O��l��
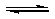
HCOOH��l��+CH
3OH��l����H��0
ijС��ͨ��ʵ���о��÷�Ӧ����Ӧ����������仯���Բ��ƣ�����Ӧ��ϵ�и���ֵ���ʼ�����±���
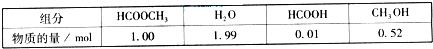
�������ת�������¶�T
1���淴Ӧʱ�䣨t���ı仯����ͼ��
��1�������������������㲻ͬʱ�䷶Χ�ڼ��������ƽ����Ӧ���ʣ�������±���

�����15��20min��Χ�ڼ�������ļ�����Ϊ
0.045
0.045
mol�����������ƽ����Ӧ����Ϊ
0.009
0.009
mol?min
-1����Ҫ��д��������̣���
��2�������������ݣ�д���÷�Ӧ�ķ�Ӧ�����ڲ�ͬ�εı仯���ɼ���ԭ��
�ٷ�Ӧ���ڣ���Ȼ������������ϴ���������С����Ч�������ԣ���Ӧ���ʽ�����
�ڷ�Ӧ���ڣ������������࣬��Ч����������Ӧ������������
�۷�Ӧ���ڣ����������ӵ�һ���̶Ⱥ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ����������أ��ر����淴Ӧ���ʵ�����ʹ�ܷ�Ӧ������С��ֱ��Ϊ�㣮
�ٷ�Ӧ���ڣ���Ȼ������������ϴ���������С����Ч�������ԣ���Ӧ���ʽ�����
�ڷ�Ӧ���ڣ������������࣬��Ч����������Ӧ������������
�۷�Ӧ���ڣ����������ӵ�һ���̶Ⱥ�Ũ�ȶԷ�Ӧ���ʵ�Ӱ����������أ��ر����淴Ӧ���ʵ�����ʹ�ܷ�Ӧ������С��ֱ��Ϊ�㣮
��
��3��������Ӧ��ƽ�ⳣ������ʽΪ��K=
| c(HCOOH)?c(CH3OH) |
| c(HCOOCH3)?c(H2O) |
����÷�Ӧ���¶�T
1�µ�KֵΪ
��
��4�������������䣬���ı��¶�ΪT
2��T
2����T
1�����ڴ����ͼ�л����¶�T
2�¼������ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��

 ��2009?�㶫���������ˮ�ⷴӦ����ʽΪ��
��2009?�㶫���������ˮ�ⷴӦ����ʽΪ�� HCOOH��l��+CH3OH��l����H��0
HCOOH��l��+CH3OH��l����H��0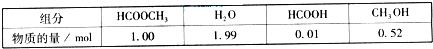

 ��
��


 ��2009?�㶫ģ�⣩�̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ�����ش��������⣺
��2009?�㶫ģ�⣩�̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ�����ش��������⣺