
ТСЦЄNH4+ + OHЈ  NH3Ўь + H2OЈ¬NH3ДЬК№КЄИуµДємЙ«КЇИпКФЦЅ±дА¶ЎЈПЦДіИЬТєЦРїЙДЬє¬УРПВБР6ЦЦАлЧУЦРµДДіјёЦЦЈєNa+ЎўNH4+ЎўK+ЎўClЈЎўSO42ЈЎўCO32ЈЎЈОЄИ·ИПИЬТєЧйіЙЅшРРИзПВКµСйЈєЈЁ1Ј©ИЎ200 mLЙПКцИЬТєЈ¬јУИлЧгБїBaCl2ИЬТєЈ¬·ґУ¦єуЅ«іБµн№эВЛЎўПґµУЎўёЙФпЈ¬µГіБµн4.30gЈ¬ПтіБµнЦРјУИл№эБїµДСОЛбЈ¬УР2.33gіБµнІ»ИЬЎЈЈЁ2Ј©ПтЈЁ1Ј©µДВЛТєЦРјУИлЧгБїµДNaOHИЬТєЈ¬јУИИЈ¬ІъЙъДЬК№КЄИуємЙ«КЇИпКФЦЅ±дА¶µДЖшМе1.12LЈЁТС»»ЛгіЙ±кЧјЧґїцЈ¬јЩ¶ЁІъЙъµДЖшМеИ«ІїТЭіцЈ©ЎЈУЙґЛїЙТФµГіц№ШУЪФИЬТєЧйіЙµДХэИ·ЅбВЫКЗ
NH3Ўь + H2OЈ¬NH3ДЬК№КЄИуµДємЙ«КЇИпКФЦЅ±дА¶ЎЈПЦДіИЬТєЦРїЙДЬє¬УРПВБР6ЦЦАлЧУЦРµДДіјёЦЦЈєNa+ЎўNH4+ЎўK+ЎўClЈЎўSO42ЈЎўCO32ЈЎЈОЄИ·ИПИЬТєЧйіЙЅшРРИзПВКµСйЈєЈЁ1Ј©ИЎ200 mLЙПКцИЬТєЈ¬јУИлЧгБїBaCl2ИЬТєЈ¬·ґУ¦єуЅ«іБµн№эВЛЎўПґµУЎўёЙФпЈ¬µГіБµн4.30gЈ¬ПтіБµнЦРјУИл№эБїµДСОЛбЈ¬УР2.33gіБµнІ»ИЬЎЈЈЁ2Ј©ПтЈЁ1Ј©µДВЛТєЦРјУИлЧгБїµДNaOHИЬТєЈ¬јУИИЈ¬ІъЙъДЬК№КЄИуємЙ«КЇИпКФЦЅ±дА¶µДЖшМе1.12LЈЁТС»»ЛгіЙ±кЧјЧґїцЈ¬јЩ¶ЁІъЙъµДЖшМеИ«ІїТЭіцЈ©ЎЈУЙґЛїЙТФµГіц№ШУЪФИЬТєЧйіЙµДХэИ·ЅбВЫКЗ
AЎўТ»¶ЁґжФЪSO42ЈЎўCO32ЈЎўNH4+Ј¬їЙДЬґжФЪNa+ЎўK+ЎўClЈ
BЎўТ»¶ЁґжФЪSO42ЈЎўCO32ЈЎўNH4+ЎўClЈЈ¬Т»¶ЁІ»ґжФЪNa+ЎўK+
CЎўc(CO32Ј)=0.01 mol/LЈ¬c(NH4+)Јѕc(SO42Ј)
DЎўИз№ыЙПКц6ЦЦАлЧУ¶јґжФЪЈ¬Фтc(ClЈ)Јѕc(SO42Ј)
D
ЎѕЅвОцЎї
КФМв·ЦОцЈєИЎЙЩБїёГИЬТєјУИлBaCl2ИЬТєУР°ЧЙ«іБµнЙъіЙЈ¬ФЩјУИлЧгБїСОЛбєуЈ¬іБµнІї·ЦИЬЅвЈ¬ІўУРЖшМеЙъіЙЈ¬ЛµГч°ЧЙ«іБµнОЄBaCO3єНBaSO4Ј¬ФтИЬТєЦРє¬УРCO32-ЎўSO42-Ј»ПтЈЁ1Ј©µДВЛТєЦРјУИлЧгБїµДNaOHИЬТєЈ¬јУИИЈ¬ІъЙъДЬК№КЄИуємЙ«КЇИпКФЦЅ±дА¶µДЖшМеЈ¬ЛµГчИЬТєЦРУРNH4+Ј»БнИЎЙПКцФИЬТєЙЩБїЈ¬µОјУУГПхЛбЛб»ЇµДПхЛбТшИЬТєЈ¬Г»УРГчПФПЦПуЈ¬ЛµГчИЬТєЦРГ»УРCl-Ј®№КИЬТєЦРТ»¶ЁґжФЪSO42-ЎўCO32-ЎўNH4+Ј¬Т»¶ЁІ»ґжФЪCl-Ј¬їЙДЬґжФЪNa+ЎўK+ЎЈіБµн4.30gОЄBaCO3єНBaSO4µДЧЬБїЈ¬УЦПтіБµнЦРјУИл№эБїµДСОЛбЈ¬УР2.33gіБµнІ»ИЬЈ¬№КBaSO4µДОпЦКµДБїОЄ(2.33g)/(233g/mol)=0.01mol, ФтBaCO3µДµДОпЦКµДБїОЄ0.01 molЈ¬c(CO32Ј)=0.01mol/0.2L=0.05 mol/L,УЦNH4+ + OHЈ  NH3Ўь + H2OЈ¬ЗТNH3µДОпЦКµДБїОЄ1.12L/(22.4L/mol)=0.05molЎЈФтc(NH4+)Јѕc(SO42Ј)ЎЈИз№ыЙПКц6ЦЦАлЧУ¶јґжФЪЈ¬ФтґжФЪИзПВЖЅєвЈєn(Na+)+ n(K+)+ n(NH4+)= n(SO42Ј) +n (CO32Ј) +n (ClЈ)Ј¬јґn(Na+)+ n(K+)+0.05mol=0.01mol+0.01mol+ n (ClЈ),№Кn (ClЈ)=0.03+ n(Na+)+ n(K+)©ѓn(SO42Ј) јґc(ClЈ)Јѕc(SO42Ј)ЎЈ
NH3Ўь + H2OЈ¬ЗТNH3µДОпЦКµДБїОЄ1.12L/(22.4L/mol)=0.05molЎЈФтc(NH4+)Јѕc(SO42Ј)ЎЈИз№ыЙПКц6ЦЦАлЧУ¶јґжФЪЈ¬ФтґжФЪИзПВЖЅєвЈєn(Na+)+ n(K+)+ n(NH4+)= n(SO42Ј) +n (CO32Ј) +n (ClЈ)Ј¬јґn(Na+)+ n(K+)+0.05mol=0.01mol+0.01mol+ n (ClЈ),№Кn (ClЈ)=0.03+ n(Na+)+ n(K+)©ѓn(SO42Ј) јґc(ClЈ)Јѕc(SO42Ј)ЎЈ
їјµгЈєіЈјыСфАлЧУµДјмСйЈ»іЈјыТхАлЧУµДјмСйЎЈ


 їмАЦРЎІ©Кї№®№МУлМбёЯПµБРґр°ё
їмАЦРЎІ©Кї№®№МУлМбёЯПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016ЅмЅЛХКЎёЯТ»ПВС§ЖЪС§Зй·ЦОцїјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ПВБР·ЦЧУЦРЛщУРФЧУ¶јВъЧгЧоНвІгОЄ8µзЧУЅб№№µДКЗ
AЈ®CCl4 BЈ®H2O CЈ®BF3 DЈ®PCl5
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016ЅмЅЛХКЎ¶«МЁКРёЯТ»ЙПС§ЖЪЖЪД©їјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
ИзНјКЗІв¶ЁВБ·ЫЈЁє¬Гѕ·ЫЈ©µДґї¶ИµДКµСйЧ°ЦГЎЈЛщУГµДNaOHЈЁЧгБїЈ©µДОпЦКµДБїЕЁ¶ИОЄ4.5 molЎ¤LЈ1ЎЈІ»Н¬К±јдµзЧУМмЖЅµД¶БКэИзПВ±нЛщКѕЈє
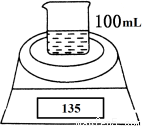
КµСйІЩЧч | К±јд/min | µзЧУМмЖЅµД¶БКэ/g |
ЙХ±Ј«NaOHИЬТє | 0 | 120 |
ЙХ±Ј«NaOHИЬТєЈ«СщЖ· | 0 | 135 |
1 | 134.5 | |
2 | 134.1 | |
3 | 133.8 | |
4 | 133.8 |
ЈЁ1Ј©·ґУ¦ЦРЙъіЙЖшМеµДЦКБї gЎЈ
ЈЁ2Ј©КФјЖЛгСщЖ·ЦРВБµДЦКБї·ЦКэЎЈ(РґіцЅвМв№эіМ)
ЈЁ3Ј©·ґУ¦єуИЬТє(ИЬТєµДМе»э±д»ЇєцВФ)µДc(OHЈ)ЎЈ(РґіцЅвМв№эіМ)
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016ЅмЅЛХКЎ¶«МЁКРёЯТ»ЙПС§ЖЪЖЪД©їјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ПВБРІЩЧч·Ѕ·Ё»тКµСйЧ°ЦГХэИ·µДКЗЈЁ Ј©
ўЩ 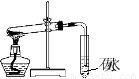 ўЪ
ўЪ  ўЫ
ўЫ 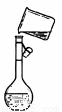 ўЬ
ўЬ 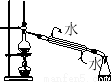
AЈ®Ч°ЦГўЩМЅѕїNaHCO3µДИИОИ¶ЁРФ
BЈ®Ч°ЦГўЪCl2µДКХјЇ
CЈ®Ч°ЦГўЫПтИЭБїЖїЦРЧЄТЖТєМе
DЈ®Ч°ЦГўЬКЇУНХфБу
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016ЅмЅЛХКЎ¶«МЁКРёЯТ»ЙПС§ЖЪЖЪД©їјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ѕшґу¶аКэФЧУєЛКЗУЙЦКЧУєНЦРЧУ№№іЙµДЈ¬Из№ыЦКЧУ»тЦРЧУОЄДіР©МШ¶ЁКэЦµЈ¬ФЧУєЛѕНТміЈОИ¶ЁЈ¬їЖС§јТЅ«ХвР©КэЦµіЖОЄЎ°»ГКэЎ±Ј¬їЖС§јТФЪИЛФм№иН¬О»ЛШ1442SiЦР·ўПЦРВµДОпАнС§Ў°»ГКэЎ±Ј¬ПВБРУР№ШЛµ·ЁХэИ·µДКЗЈЁ Ј©
AЈ®1442SiФЧУєЛДЪє¬УР42ёцЦРЧУ BЈ®№иУГУЪЦЖФм№вµјПЛО¬
CЈ®¶юСх»Ї№иУГУЪ°лµјМеІДБП DЈ®SiO2їЙТФУлИхЛбHF·ґУ¦
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016ЅмЅЛХДПѕ©КРёЯТ»ЙПС§ЖЪЖЪЦРїјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ФЪpHЈЅ1µДОЮЙ«ИЬТєЦРДЬґуБї№ІґжµДАлЧУЧйКЗ
AЈ®NH4Ј«ЎўMg2Ј«ЎўSO42ЈЎўClЈ BЈ®Ba2Ј«ЎўKЈ«ЎўOHЈЎўNO3Ј
CЈ®Al3Ј«ЎўCu2Ј«ЎўSO42ЈЎўClЈ DЈ®NaЈ«ЎўCa2Ј«ЎўClЈЎўCO32Ј
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016ЅмЅЛХДПѕ©КРёЯТ»ЙПС§ЖЪЖЪЦРїјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ФЪКўУРµвЛ®µДИэЦ§КФ№ЬЦР·Ц±рјУИл±ЅЎўЛДВИ»ЇМјєНѕЖѕ«Ј¬ХсµґєуѕІЦГЈ¬іцПЦИзНјЛщКѕПЦПуЈ¬ФтјУИлµДКФјБ·Ц±рКЗ
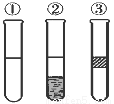
AЈ®ўЩКЗѕЖѕ«Ј¬ўЪКЗCCl4Ј¬ўЫКЗ±Ѕ
BЈ®ўЩКЗ±ЅЈ¬ўЪКЗCCl4Ј¬ўЫКЗѕЖѕ«
CЈ®ўЩКЗCCl4Ј¬ўЪКЗ±ЅЈ¬ўЫКЗѕЖѕ«
DЈ®ўЩКЗ±ЅЈ¬ўЪКЗѕЖѕ«Ј¬ўЫКЗCCl4
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016Ѕм№г¶«КЎёЯТ»ЙПС§ЖЪЖЪЦРїјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ПаН¬ЦКБїµДSO2єНSO3ЛьГЗЦ®јдµД№ШПµХэИ·µДКЗЈЁ Ј©
AЈ®Лщє¬БтФЧУµДОпЦКµДБїЦ®±ИОЄ1:1 BЈ®СхФЧУµДОпЦКµДБїЦ®±ИОЄ5:6
CЈ®СхФЄЛШµДЦКБї±ИОЄ2:3 DЈ®БтФЄЛШµДЦКБї±ИОЄ5:4
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2016Ѕм№г¶«КЎёЯТ»ЙПС§ЖЪЖЪЦРїјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєСЎФсМв
ПВБРИЬТєЦРµДВИАлЧУКэДїУл50 mL 1 mol/LµДAlCl3ИЬТєЦРВИАлЧУКэДїПаµИµДКЗ
AЈ®75 mL 2 mol/LµДCaCl2 BЈ®150 mL 1 mol/LµДNaCl
CЈ®150 mL 3 mol/LµДKCl DЈ®100 mL 2 mol/LµДNH4Cl
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com