

| A�������� | B���ձ� | C��©�� | D��250mL����ƿ |


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

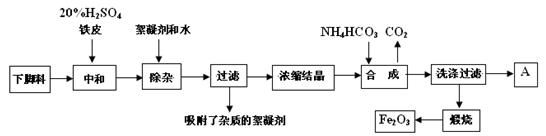
 ��ʾ��ͼ�ڷ����ڻ�����ϩ�ķ���װ��(�г�װ�ò��ػ�������Ҫ���ȵ������·��á����)��
��ʾ��ͼ�ڷ����ڻ�����ϩ�ķ���װ��(�г�װ�ò��ػ�������Ҫ���ȵ������·��á����)�� ��������̣�ʵ��ʱ��ҪѸ��Ϩ����棬�����ȫ�IJ��������ǣ� ��
��������̣�ʵ��ʱ��ҪѸ��Ϩ����棬�����ȫ�IJ��������ǣ� ��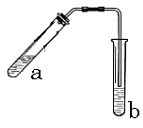
 ��ʵ���������������Ļ�ѧ����ʽ(Ҫ�����-18ʾ��ԭ��)
��ʵ���������������Ļ�ѧ����ʽ(Ҫ�����-18ʾ��ԭ��)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO2 | B��H2 | C��O2 | D��NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
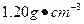 )����100 mL 0.200
)����100 mL 0.200 ����ʱ��������ʽ�ζ�����ȡ24.5������_______ mL,ϴ������ʽ�ζ���Ӧ�� ����������ȡ��������ᡣ
����ʱ��������ʽ�ζ�����ȡ24.5������_______ mL,ϴ������ʽ�ζ���Ӧ�� ����������ȡ��������ᡣ ��������Һ�ⶨ����������Ʒ�������Ȼ��Ƶĺ�����
��������Һ�ⶨ����������Ʒ�������Ȼ��Ƶĺ�����| ʵ���� | �ζ���ʼʱ�ζ��ܵĶ���Ϊ | �ζ����ʱ���ζ��ܵĶ���Ϊ | ������Һ����� ��mL�� |
| 1 | 0.07 | 22.59 | 20.00 |
| 2 | 0.22 | 22.72 | 20.00 |
| 3 | 0.35 | 22.83 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
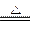 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O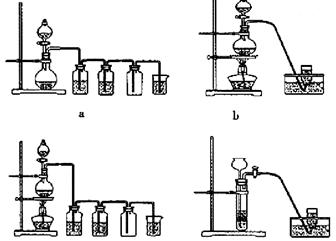
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com