
��12�֣�����������������Ӧ����Ϊ�㷺�������Բ���֮һ����������¼���ϣ���������ϣ����������Ӳ��ϵȡ��������\�������ҽѧ����Ҳ�кܺõ�Ӧ��ǰ��������������Ŀǰ�Ʊ�������������������Ҫ��������������ͼʾ��

��ش��������⣺��1��ʵ���ұ�����ҺAʱ������� ��
��2��Ϊ�˵õ��ϴ�������Fe3O4��FeSO4��7H2O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ ���ڴ������£�������Ԫ���Ƿ������ȫ��ʵ������� ��
��3��д���Ʊ�����Fe3O4�����ӷ�Ӧ����ʽ ��
��4��������������Ҫ�����ǣ��ٵõ���Fe3O4�������Ӽ�����ž�����
�� ��
��5���������ǵ��Ӳ��ϵ����㣬���Ⱦ��й���Ĵ��ԣ��־���Һ��������ԣ����й�������Fe3O4�������˵���в���ȷ���ǣ� ��
A������Fe3O4�������ɢϵ������Һ��
B������Fe3O4���������ͨ���������õ��ᴿ��
C����һ���ɼ���ͨ���ô�����ʱ����ֹ�����ͨ·��
D������Fe3O4������Ƚ��ȶ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ������ѧ����12��������⻯ѧ�Ծ����������� ���ͣ���ѡ��
����������������Ӧ����Ϊ�㷺�������Բ���֮һ������������Ŀǰ�Ʊ�������������������Ҫ��������������ͼʾ��
����˵������ȷ����
| A��������ҺAʱ��Ӧ������������ |
| B�����Ʊ���Ӧ���Ͳ�����������ԭ��Ӧ |
| C��ȡ��Һ������ɫ��Ӧ������Ϊ��ɫ |
| D��FeSO4��7H2O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ����12��������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����������������Ӧ����Ϊ�㷺�������Բ���֮һ������������Ŀǰ�Ʊ�������������������Ҫ��������������ͼʾ��

����˵������ȷ����
A��������ҺAʱ��Ӧ������������
B�����Ʊ���Ӧ���Ͳ�����������ԭ��Ӧ
C��ȡ��Һ������ɫ��Ӧ������Ϊ��ɫ
D��FeSO4��7H2O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�����������������Ӧ����Ϊ�㷺�������Բ���֮һ����������¼���ϣ���������ϣ����������Ӳ��ϵȡ��������\�������ҽѧ����Ҳ�кܺõ�Ӧ��ǰ��������������Ŀǰ�Ʊ�������������������Ҫ��������������ͼʾ��
��ش��������⣺��1��ʵ���ұ�����ҺAʱ������� ��
��2��Ϊ�˵õ��ϴ�������Fe3O4��FeSO4��7H2O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ ���ڴ������£�������Ԫ���Ƿ������ȫ��ʵ������� ��
��3��д���Ʊ�����Fe3O4�����ӷ�Ӧ����ʽ ��
��4��������������Ҫ�����ǣ��ٵõ���Fe3O4�������Ӽ�����ž�����
�� ��
��5���������ǵ��Ӳ��ϵ����㣬���Ⱦ��й���Ĵ��ԣ��־���Һ��������ԣ����й�������Fe3O4�������˵���в���ȷ���ǣ� ��
A������Fe3O4�������ɢϵ������Һ��
B������Fe3O4���������ͨ���������õ��ᴿ��
C����һ���ɼ���ͨ���ô�����ʱ����ֹ�����ͨ·��
D������Fe3O4������Ƚ��ȶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�����и�����ѧ��������У������ѧ�Ծ� ���ͣ������
��12�֣�����������������Ӧ����Ϊ�㷺�������Բ���֮һ����������¼���ϣ���������ϣ����������Ӳ��ϵȡ��������\�������ҽѧ����Ҳ�кܺõ�Ӧ��ǰ��������������Ŀǰ�Ʊ�������������������Ҫ��������������ͼʾ��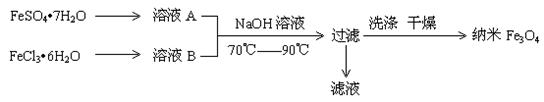
��ش��������⣺��1��ʵ���ұ�����ҺAʱ������� ��
��2��Ϊ�˵õ��ϴ�������Fe3O4��FeSO4��7H2 O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ ���ڴ������£�������Ԫ���Ƿ������ȫ��ʵ������� ��
O �� FeCl3��6H2O�����ʵ���֮�����ӦΪ ���ڴ������£�������Ԫ���Ƿ������ȫ��ʵ������� ��
��3��д���Ʊ�����Fe3O4�����ӷ�Ӧ����ʽ ��
��4��������������Ҫ�����ǣ��ٵõ���Fe3O4�������Ӽ�����ž�����
�� ��
��5���������ǵ��Ӳ��ϵ����㣬���Ⱦ��й���Ĵ��ԣ��־���Һ��������ԣ����й�������Fe3O4�������˵���в���ȷ���ǣ� ��
| A������Fe3O4�������ɢϵ������Һ�� |
B������Fe3O4������� ��ͨ���������õ��ᴿ�� ��ͨ���������õ��ᴿ�� |
| C����һ���ɼ���ͨ���ô�����ʱ����ֹ�����ͨ·�� |
| D������Fe3O4������Ƚ��ȶ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com