



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ��Ҧ��ѧ2011��2012ѧ��߶���ѧ�ڵ�һ���ʼ컯ѧ����(ʵ���) ���ͣ�058
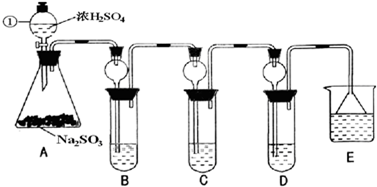
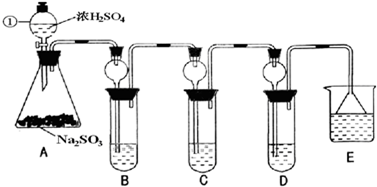
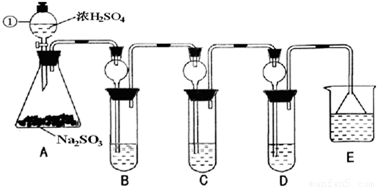
ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����
(1)
��B�м���S02�������ԣ���B����ʢ�Լ���Ϊ________��(2)��C��װFeCl3��Һ������SO2�Ļ�ԭ�ԣ���C�з�Ӧ�����ӷ���ʽΪ________��
(3)��D��װ����ijŨ��Һ��ͨ��SO2���΅����D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷֽ�����̽������ش���������
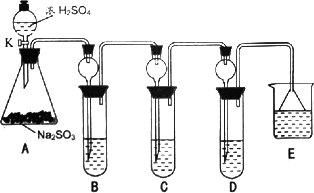
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5 mol��L��1��HCl��0.5 mol��L��1��H2S04��0.5 mol��L��1��BaCl2��Ʒ����Һ�����ƳΜ[ʯ��ˮ��
(1)����һ���ð�ɫ����ΪCaSO3
��������ð�ɫ����Ϊ________
���������ð�ɫ����Ϊ�����������ʵĻ���
(ii)���ڼ���һ����д�±���

(iii)���������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
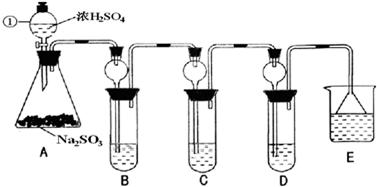
��Ŀ�����л�ѧ ��Դ��2012�������ʮ�����ص�ѧУ�����߿���ѧģ���Ծ���һ���������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com