
(1) œ¬¡–”–πÿ µ—È≤Ÿ◊˜ªÚ≈–∂œ≤ª’˝»∑µƒ « _________
A£Æ”√10 mL¡øÕ≤¡ø»°œ°¡ÚÀ·»Ð“∫ 8.0 mL B£Æ”√∏…‘ÔµƒpH ‘÷Ω≤‚∂®¬»ÀƵƒpH
C£Æ”√ºÓ ΩµŒ∂®πСø»°KMnO4»Ð“∫ 19.60 mL
D£Æ π”√»ð¡ø∆ø≈‰÷∆»Ð“∫ ±£¨∏© ”“∫√Ê∂®»ð∫ÛÀ˘µ√»Ð“∫µƒ≈®∂»∆´¥Û
E£Æ‘≤µ◊…’∆ø°¢◊∂–Œ∆ø°¢’Ù∑¢√Ûº”»» ±∂º”¶µÊ‘⁄ Ø√ÞÕ¯…œ
£®2£©∏˘æð”“Õº√Ë ˆªÿ¥œ¬¡–Œ £∫
¢Ÿπÿ±’ÕºA◊∞÷√÷–µƒ÷πÀƺ–a∫Û£¨¥”≥§æ±¬©∂∑œÚ ‘πÐ÷–◊¢»Î“ª∂®¡øµƒÀÆ£¨æ≤÷√∫Û»ÁÕºÀ˘ æ°£ ‘≈–∂œ£∫A◊∞÷√ «∑Ò¬©∆¯£ø(ÃÓ–Ú∫≈) 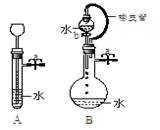
A.¬©∆¯ B.≤ª¬©∆¯ C.≤ªƒÐ»∑∂®
¢⁄πÿ±’ÕºB◊∞÷√÷–µƒ÷πÀƺ–a∫Û£¨ø™∆ÙªÓ»˚b£¨ÀÆ≤ª∂œÕ˘œ¬µŒ£¨÷±÷¡»´≤ø¡˜»Î…’∆ø°£ ‘≈–∂œ£∫B◊∞÷√ «∑Ò¬©∆¯£ø(ÃÓ–Ú∫≈) A.¬©∆¯ B.≤ª¬©∆¯ C.≤ªƒÐ»∑∂®
 ‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
A.∏Ω◊≈”⁄ ‘πЃ⁄±⁄µƒ±Ω∑”£¨ø…”√ºÓ“∫œ¥µ”
B.”√Ω·æß∑®ø…“‘≥˝»•œıÀ·ºÿ÷–ªÏ”–µƒ…Ÿ¡ø¬»ªØƒ∆
C.«–∏Ó∞◊¡◊ ±£¨±ÿ–Δ√ƒ˜◊”º–»°£¨÷√”⁄◊¿√Ê…œµƒ≤£¡ß∆¨…œ£¨–°–ƒ”√µ∂«–∏Ó
D. µ—È ±£¨≤ª…˜¥Ú∑≠»º◊≈µƒæ∆æ´µ∆£¨ø…¡¢º¥”√ ™ƒ®≤º∏«√ª—Ê
E.≈®¡ÚÀ·≤ª–°–ƒ’¥µΩ∆§∑Ù…œ£¨¡¢øÔ√œ°…’ºÓ»Ð“∫œ¥µ”
F.‘⁄«‚—ıªØÃ˙Ω∫ÃÂ÷–µŒº”…Ÿ¡øœ°¡ÚÀ·ª·≤˙…˙≥¡µÌ
(2)œ¬±Ì «ƒ≥÷÷≥£º˚Ω Ùµƒ≤ø∑÷–‘÷ £∫
—’…´◊¥Ã¨ | ”≤∂» | √Ð∂» | »€µ„ | µºµÁ–‘ | µº»»–‘ | —”’π–‘ |
“¯∞◊…´πÃà| Ωœ»Ì | 2.70 g°§cm-3 | 660.4 °Ê | ¡º∫√ | ¡º∫√ | ¡º∫√ |
Ω´∏√Ω ÙÕ∂»Î¿‰ÀÆ÷–ŒÞ±‰ªØ£¨Õ∂»Îœ°—ŒÀ·÷–ø…≤˙…˙¥Û¡øµƒŒÞ…´∆¯Ã°£ ‘ªÿ¥£∫
¢ŸÕ∆∂œ∏√Ω Ùø…ƒÐµƒ“ª÷÷”√Õæ__________£¨∏√Ω ÙµƒªÓ∂Ø–‘±»Õ≠__________(ÃÓ°∞«ø°±ªÚ°∞»ı°±)°£
¢⁄«Î◊‘—° ‘º¡£¨…˺∆≤ªÕ¨µƒ µ—ÈÃΩæø∏√Ω Ù”ÎÃ˙µƒªÓ∂Ø–‘«ø»ı£¨≤¢ÕÍ≥…œ¬±Ì£∫
≤¬œÎ | —È÷§∑Ω∑® | ‘§≤‚ µ—Èœ÷œÛ |
∏√Ω Ù±»Ã˙ªÓ∆√ |
|
|
∏√Ω ÙªÓ∆√–‘±»Ã˙»ı |
|
|
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
A.∏Ω◊≈”⁄ ‘πЃ⁄±⁄µƒ±Ω∑”£¨ø…”√ºÓ“∫œ¥µ”
B.”√Ω·æß∑®ø…“‘≥˝»•œıÀ·ºÿ÷–ªÏ”–µƒ…Ÿ¡ø¬»ªØƒ∆
C.«–∏Ó∞◊¡◊ ±£¨±ÿ–Δ√ƒ˜◊”º–»°£¨÷√”⁄◊¿√Ê…œµƒ≤£¡ß∆¨…œ£¨–°–ƒ”√µ∂«–∏Ó
D. µ—È ±£¨≤ª…˜¥Ú∑≠»º◊≈µƒæ∆æ´µ∆£¨ø…¡¢º¥”√ ™ƒ®≤º∏«√ª—Ê
E.≈®¡ÚÀ·≤ª–°–ƒ’¥µΩ∆§∑Ù…œ£¨¡¢øÔ√œ°…’ºÓ»Ð“∫œ¥µ”
F.‘⁄«‚—ıªØÃ˙Ω∫ÃÂ÷–µŒº”…Ÿ¡øœ°¡ÚÀ·ª·≤˙…˙≥¡µÌ
(2)œ¬±Ì «ƒ≥÷÷≥£º˚Ω Ùµƒ≤ø∑÷–‘÷ £∫
—’…´◊¥Ã¨ | ”≤∂» | √Ð∂» | »€µ„ | µºµÁ–‘ | µº»»–‘ | —”’π–‘ |
“¯∞◊…´πÃà| Ωœ»Ì | 2.70 g°§cm-3 | 660.4 °Ê | ¡º∫√ | ¡º∫√ | ¡º∫√ |
Ω´∏√Ω ÙÕ∂»Î¿‰ÀÆ÷–ŒÞ±‰ªØ£¨Õ∂»Îœ°—ŒÀ·÷–ø…≤˙…˙¥Û¡øµƒŒÞ…´∆¯Ã°£«Îªÿ¥°£
¢ŸÕ∆∂œ∏√Ω Ùø…ƒÐµƒ“ª÷÷”√Õæ_______£¨∏√Ω ÙµƒªÓ∂Ø–‘±»Õ≠_______(ÃÓ°∞«ø°±ªÚ°∞»ı°±)°£
¢⁄«Î◊‘—° ‘º¡£¨…˺∆≤ªÕ¨µƒ µ—ÈÃΩæø∏√Ω Ù”ÎÃ˙µƒªÓ∂Ø–‘«ø»ı£¨≤¢ÕÍ≥…œ¬±Ì£∫
≤¬œÎ | —È÷§∑Ω∑® | ‘§≤‚ µ—Èœ÷œÛ |
∏√Ω Ù±»Ã˙ªÓ∆√ |
|
|
∏√Ω ÙªÓ∆√–‘±»Ã˙»ı |
|
|
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014ΩÏ…Ω∂´ °∏þ∂˛ ÓºŸ◊˜“µ£®“ª£©ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫ µ—ÈÂ
ªØ—ß µ—È «—–æøŒÔ÷ –‘÷ µƒª˘¥°°£
(1)œ¬¡–”–πÿ µ—È≤Ÿ◊˜ªÚ≤‚¡ø ˝æð∫œ¿Ìµƒ «________(ÃÓ–Ú∫≈)°£
a£Æ”√Ã˙€·€ˆº”»»CuSO4°§5H2OæßÃÂ≤‚∂®Ω·æßÀÆ÷ ¡ø∑÷ ˝
b£Æ”√∏…‘ÔµƒpH ‘÷Ω≤‚∂®≈®¡ÚÀ·µƒpH
c£Æ”√πÊ∏ÒŒ™20 mLµƒ¡øÕ≤£¨¡ø»°16.8 mLµƒNa2CO3»Ð“∫
(2)ƒ≥∑œÀÆ—˘∆∑÷–∫¨”–“ª∂®¡øµƒNa£´°¢CO32-°¢SO32-£¨ƒ≥—–æø–°◊È”˚≤‚∂®∆‰÷–SO32-µƒ≈®∂»°£
µ—È∑Ω∞∏£∫
¢°.”√…’±≠ ¢»°∑œÀÆ ¡ø£¨º”…Ÿ¡øªÓ–‘Ãø£¨≥˝»•∑œÀÆ÷–µƒ‘”÷ £ªπ˝¬À£¨»°¬À“∫£ª
¢¢.æ´»∑¡ø»°20.00 mLπ˝¬À∫Û∑œÀÆ ‘—˘£¨—°‘Ò π”√◊œ…´µƒ0.1 mol/L KMnO4(H2SO4À·ªØ)»Ð“∫Ω¯––µŒ∂®£ª
¢£.º«¬º ˝æ𣨺∆À„°£
¢Ÿœ¬¡–µŒ∂®∑Ω Ω÷–£¨◊Ó∫œ¿Ìµƒ «(º–≥÷≤ø“—∑÷¬‘»•)______(ÃÓ◊÷ƒ∏–Ú∫≈)°£

¢⁄µŒ∂®π˝≥Ã÷–£¨”–πÿ∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω «__________________________________°£
(3)ƒ≥Õ¨—ß÷∆±∏Fe(OH)3Ω∫ã∫”√Ωý檵ƒ…’±≠»° ¡ø’Ù¡ÛÀƺ”»»÷¡∑–Ã⁄£¨œÚ…’±≠÷–µŒº” 1 mol/LµƒFeCl3»Ð“∫£¨≤¢≤ª∂œ”√≤£¡ß∞ÙΩ¡∞Ë£¨Ω·π˚»Ð“∫±‰ªÎ◊«°£∏√Õ¨—ß÷∆±∏Ω∫àß∞еƒ‘≠“Ú «°°°°°°°°°°°°°°£¨ƒ„»œŒ™≥…π¶÷∆µ√Fe(OH)3Ω∫õƒÃÿ’˜œ÷œÛ «_________°£
(4)”√œ¬Õº◊∞÷√Ω¯––CO2–‘÷ µƒ”–πÿ µ—È°£

‘º¡∆øB÷– ¢”–±•∫ÕNaHCO3»Ð“∫£¨∆‰ƒøµƒ «:
_______________________ __________°£
¢⁄∑¥”¶π˝≥Ã÷–£¨E÷–≥Œ«Â ت“ÀƱ‰ªÎ◊«£¨E÷–µƒªÏ∫œÃÂœµ÷–≥˝¥Ê‘⁄µÁ¿Î∆Ω∫‚°¢ÀÆΩ‚∆Ω∫‚Õ‚£¨ªπ¥Ê‘⁄»ÐΩ‚∆Ω∫‚£¨”√∑Ω≥Ã Ω±Ì æ∏√»ÐΩ‚∆Ω∫‚πÿœµ£∫
____________________ ___________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2010ƒÍ∞≤«÷–—ß∏þ∂˛ Ó∆⁄¡∑œ∞ªØ—ßæÌ£®¡˘£© –գ∫ÃÓø’Â
(1) œ¬¡–”–πÿ µ—È≤Ÿ◊˜ªÚ≈–∂œ≤ª’˝»∑µƒ « _________
A£Æ”√10 mL¡øÕ≤¡ø»°œ°¡ÚÀ·»Ð“∫ 8.0 mL B£Æ”√∏…‘ÔµƒpH ‘÷Ω≤‚∂®¬»ÀƵƒpH
C£Æ”√ºÓ ΩµŒ∂®πСø»°KMnO4»Ð“∫ 19.60 mL
D£Æ π”√»ð¡ø∆ø≈‰÷∆»Ð“∫ ±£¨∏© ”“∫√Ê∂®»ð∫ÛÀ˘µ√»Ð“∫µƒ≈®∂»∆´¥Û
E£Æ‘≤µ◊…’∆ø°¢◊∂–Œ∆ø°¢’Ù∑¢√Ûº”»» ±∂º”¶µÊ‘⁄ Ø√ÞÕ¯…œ
£®2£©∏˘æð”“Õº√Ë ˆªÿ¥œ¬¡–Œ £∫
¢Ÿπÿ±’ÕºA◊∞÷√÷–µƒ÷πÀƺ–a∫Û£¨¥”≥§æ±¬©∂∑œÚ ‘πÐ÷–◊¢»Î“ª∂®¡øµƒÀÆ£¨æ≤÷√∫Û»ÁÕºÀ˘ æ°£ ‘≈–∂œ£∫A◊∞÷√ «∑Ò¬©∆¯£ø(ÃÓ–Ú∫≈)
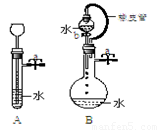
A.¬©∆¯ B.≤ª¬©∆¯ C.≤ªƒÐ»∑∂®
¢⁄πÿ±’ÕºB◊∞÷√÷–µƒ÷πÀƺ–a∫Û£¨ø™∆ÙªÓ»˚b£¨ÀÆ≤ª∂œÕ˘œ¬µŒ£¨÷±÷¡»´≤ø¡˜»Î…’∆ø°£ ‘≈–∂œ£∫B◊∞÷√ «∑Ò¬©∆¯£ø(ÃÓ–Ú∫≈) A.¬©∆¯ B.≤ª¬©∆¯ C.≤ªƒÐ»∑∂®
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com